We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1712
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
=Anaplastic Lymphoma Kinase receptor= | =Anaplastic Lymphoma Kinase receptor= | ||
<StructureSection load='7n00' size='350' frame='true'side='right' caption='Anaplastic Lymphoma Kinase receptor PDB code: [https://www.rcsb.org/structure/7N00 7n00]' scene='90/904317/Dimer_full_colored/8'> | <StructureSection load='7n00' size='350' frame='true'side='right' caption='Anaplastic Lymphoma Kinase receptor PDB code: [https://www.rcsb.org/structure/7N00 7n00]' scene='90/904317/Dimer_full_colored/8'> | ||
| - | + | ||
==Background== | ==Background== | ||
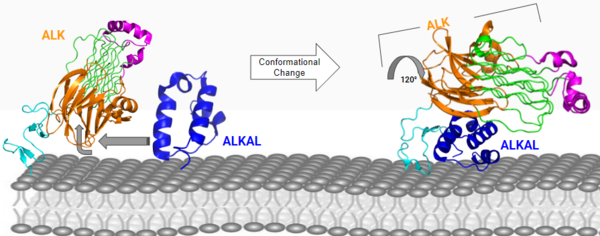
The anaplastic lymphoma kinase (ALK) was first discovered in 1994 as a tyrosine [https://en.wikipedia.org/wiki/Kinase kinase] in [https://en.wikipedia.org/wiki/Anaplastic_large-cell_lymphoma anaplastic large-cell lymphoma] (ALCL) cells.<ref>DOI: 10.3390/ijms19113448</ref> The specific type of tyrosine kinase ALK is classified as is a [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase] (RTK) and like other RTKs, it's an integral protein with extracellular and intracellular domains and is involved in transmembrane signaling and communication within the cell. ALK is commonly expressed in the development of the nervous system. Anaplastic lymphoma kinase receptor (ALKr) is the extracellular portion of the RTK that includes a binding surface for a ligand to bind. When the ALK activating ligand (ALKAL) binds to ALKr, this causes a conformational change of ALK, allowing two ALK-ALKAL complexes to interact with each other, which will then allow intracellular kinase domain of ALK to phosphorylate a tyrosine residue on a downstream enzyme, which will activate this enzyme and activate a signaling cascade. Abnormal forms of ALK are closely related to the formation of several cancers. <ref>DOI: 10.3390/ijms19113448</ref> | The anaplastic lymphoma kinase (ALK) was first discovered in 1994 as a tyrosine [https://en.wikipedia.org/wiki/Kinase kinase] in [https://en.wikipedia.org/wiki/Anaplastic_large-cell_lymphoma anaplastic large-cell lymphoma] (ALCL) cells.<ref>DOI: 10.3390/ijms19113448</ref> The specific type of tyrosine kinase ALK is classified as is a [https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase receptor tyrosine kinase] (RTK) and like other RTKs, it's an integral protein with extracellular and intracellular domains and is involved in transmembrane signaling and communication within the cell. ALK is commonly expressed in the development of the nervous system. Anaplastic lymphoma kinase receptor (ALKr) is the extracellular portion of the RTK that includes a binding surface for a ligand to bind. When the ALK activating ligand (ALKAL) binds to ALKr, this causes a conformational change of ALK, allowing two ALK-ALKAL complexes to interact with each other, which will then allow intracellular kinase domain of ALK to phosphorylate a tyrosine residue on a downstream enzyme, which will activate this enzyme and activate a signaling cascade. Abnormal forms of ALK are closely related to the formation of several cancers. <ref>DOI: 10.3390/ijms19113448</ref> | ||
== Structure & Function == | == Structure & Function == | ||
===Domains=== | ===Domains=== | ||
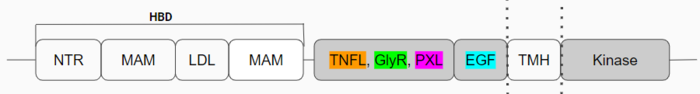
| - | [[Image:Updated schematic.png|700 px|center|thumb|Figure | + | [[Image:Updated schematic.png|700 px|center|thumb|Figure 1: Anaplastic Lymphoma Kinase and its domains.]] |
ALKr is in its inactive state as a <scene name='90/904317/Glycinerichmonomer/12'>monomer</scene> and has many different domains (Figure 1) that are important to the formation of the <scene name='90/904317/Dimer_full_colored/12'>dimerized</scene> active state, leading to ALK's main function. The [https://en.wikipedia.org/wiki/Tumor_necrosis_factor tumor necrosis factor]-like domain (TNFL), glycine-rich domain (GlyR), polyglycine extension loop (PXL), and [https://en.wikipedia.org/wiki/Growth_factor growth factor]-like domain (EGF) are the main domains of ALKr, and the only domains whose structures have been fully discovered are in color (Figure 1). The <scene name='90/904317/Glycinerichmonomer/11'>EGF</scene> (cyan) is the domain that binds to the TMH (transmembrane region), connecting the extracellular portion of ALK to the intracellular kinase domain. <scene name='90/904317/Glycinerichmonomer/10'>TNFL</scene> (orange) has a beta-sandwich structure that provides important residues that act as the binding surface for the ligand. <scene name='90/904317/Glycinerichmonomer/8'>GlyR</scene> (green) contains 14 rare polyglycine helices that are hydrogen-bound to each other. The <scene name='90/904317/Glycinerichdomain/4'>hexagonal orientation</scene> of these rare helices create a rigid structure which allows it to function as a scaffold to anchor the ligand-binding site on the TNF-like domain while bound to the ligand. The numerous hydrogen bonds are what create this <scene name='90/904317/Glycinerichdomain/3'>formation</scene> of the helices and the rigidity of the structure. The <scene name='90/904317/Glycinerichmonomer/9'>PXL</scene> (pink) connects two of these polyglycine helices, and it also plays a role in forming important interactions of the dimerized activated state of ALKr. | ALKr is in its inactive state as a <scene name='90/904317/Glycinerichmonomer/12'>monomer</scene> and has many different domains (Figure 1) that are important to the formation of the <scene name='90/904317/Dimer_full_colored/12'>dimerized</scene> active state, leading to ALK's main function. The [https://en.wikipedia.org/wiki/Tumor_necrosis_factor tumor necrosis factor]-like domain (TNFL), glycine-rich domain (GlyR), polyglycine extension loop (PXL), and [https://en.wikipedia.org/wiki/Growth_factor growth factor]-like domain (EGF) are the main domains of ALKr, and the only domains whose structures have been fully discovered are in color (Figure 1). The <scene name='90/904317/Glycinerichmonomer/11'>EGF</scene> (cyan) is the domain that binds to the TMH (transmembrane region), connecting the extracellular portion of ALK to the intracellular kinase domain. <scene name='90/904317/Glycinerichmonomer/10'>TNFL</scene> (orange) has a beta-sandwich structure that provides important residues that act as the binding surface for the ligand. <scene name='90/904317/Glycinerichmonomer/8'>GlyR</scene> (green) contains 14 rare polyglycine helices that are hydrogen-bound to each other. The <scene name='90/904317/Glycinerichdomain/4'>hexagonal orientation</scene> of these rare helices create a rigid structure which allows it to function as a scaffold to anchor the ligand-binding site on the TNF-like domain while bound to the ligand. The numerous hydrogen bonds are what create this <scene name='90/904317/Glycinerichdomain/3'>formation</scene> of the helices and the rigidity of the structure. The <scene name='90/904317/Glycinerichmonomer/9'>PXL</scene> (pink) connects two of these polyglycine helices, and it also plays a role in forming important interactions of the dimerized activated state of ALKr. | ||
Revision as of 20:32, 18 April 2022
Anaplastic Lymphoma Kinase receptor
| |||||||||||
References
- ↑ Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ Murray PB, Lax I, Reshetnyak A, Ligon GF, Lillquist JS, Natoli EJ Jr, Shi X, Folta-Stogniew E, Gunel M, Alvarado D, Schlessinger J. Heparin is an activating ligand of the orphan receptor tyrosine kinase ALK. Sci Signal. 2015 Jan 20;8(360):ra6. doi: 10.1126/scisignal.2005916. PMID:25605972 doi:http://dx.doi.org/10.1126/scisignal.2005916
- ↑ Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ De Munck S, Provost M, Kurikawa M, Omori I, Mukohyama J, Felix J, Bloch Y, Abdel-Wahab O, Bazan JF, Yoshimi A, Savvides SN. Structural basis of cytokine-mediated activation of ALK family receptors. Nature. 2021 Oct 13. pii: 10.1038/s41586-021-03959-5. doi:, 10.1038/s41586-021-03959-5. PMID:34646012 doi:http://dx.doi.org/10.1038/s41586-021-03959-5
Student Contributors
- Drew Peters
- Hillary Kulavic