We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1715
From Proteopedia
(Difference between revisions)
| Line 49: | Line 49: | ||
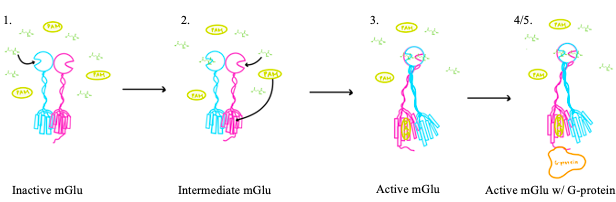
'''2.''' In the intermediate activation state, also known as the open-closed conformation, one glutamate is bound in one binding pocket of VFT. This single <scene name='90/904320/Mglu_binding/8'>glutamate bound state</scene> is still considered inactive as the receptor has not changed the conformations in the CRD and thus the TMD. With the same asymmetric transmembrane helices formation, a <scene name='90/904319/Inactive_tmd_interface/7'>TM3/TM4 interface</scene> is still present and mGlu cannot interact with a G protein<ref name="Seven">PMID:34194039</ref>. | '''2.''' In the intermediate activation state, also known as the open-closed conformation, one glutamate is bound in one binding pocket of VFT. This single <scene name='90/904320/Mglu_binding/8'>glutamate bound state</scene> is still considered inactive as the receptor has not changed the conformations in the CRD and thus the TMD. With the same asymmetric transmembrane helices formation, a <scene name='90/904319/Inactive_tmd_interface/7'>TM3/TM4 interface</scene> is still present and mGlu cannot interact with a G protein<ref name="Seven">PMID:34194039</ref>. | ||
| - | [[Image:Screen Shot 2022-04-15 at 3.54.32 PM.png|300 px|right|thumb|Figure 4. The interaction between an active mGlu (magenta/lime/purple/crimson) and a G-protein (orange) ]] | + | [[Image:Screen Shot 2022-04-15 at 3.54.32 PM.png|300 px|right|thumb|Figure 4. The interaction between an active mGlu (magenta/lime/purple/crimson) and a G-protein (orange).]] |
'''3.''' A second glutamate then binds to the other <scene name='90/904320/Active_site_interactions/3'>binding pocket</scene> of the VFT. Mediated by L639, F643, N735, W773, and F776, a <scene name='90/904320/Pam/5'>positive allosteric modulator</scene> (PAM) also binds within the seven TMD helices of the alpha chain <ref name="Seven">PMID:34194039</ref>. This closed conformation of the VFT now has an inter-lobe angle of 25° is considered to be in the <scene name='90/904320/Active_mglu/6'>active conformation</scene><ref name="Seven">PMID:34194039</ref>. The binding of these ligands allows the CRDs to compact and come together. This transformation causes the TMD to form a separate, active asymmetric conformation with a <scene name='90/904319/Active_helices/23'>TM6-TM6 interface</scene> between the chains<ref name="Seven">PMID:34194039</ref>. | '''3.''' A second glutamate then binds to the other <scene name='90/904320/Active_site_interactions/3'>binding pocket</scene> of the VFT. Mediated by L639, F643, N735, W773, and F776, a <scene name='90/904320/Pam/5'>positive allosteric modulator</scene> (PAM) also binds within the seven TMD helices of the alpha chain <ref name="Seven">PMID:34194039</ref>. This closed conformation of the VFT now has an inter-lobe angle of 25° is considered to be in the <scene name='90/904320/Active_mglu/6'>active conformation</scene><ref name="Seven">PMID:34194039</ref>. The binding of these ligands allows the CRDs to compact and come together. This transformation causes the TMD to form a separate, active asymmetric conformation with a <scene name='90/904319/Active_helices/23'>TM6-TM6 interface</scene> between the chains<ref name="Seven">PMID:34194039</ref>. | ||
Revision as of 00:55, 19 April 2022
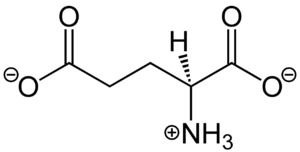
Metabotropic Glutamate Receptor
| |||||||||||
3D Structures
7epa, mGlu Inactive
7mtr, mGlu Active
7mts, mGlu Active G Protein Bound
References
- ↑ Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol. 2013;53:531-56. doi:, 10.1146/annurev-pharmtox-032112-135923. Epub 2012 Nov 8. PMID:23140243 doi:http://dx.doi.org/10.1146/annurev-pharmtox-032112-135923
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295-322. doi:, 10.1146/annurev.pharmtox.011008.145533. PMID:20055706 doi:http://dx.doi.org/10.1146/annurev.pharmtox.011008.145533
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 Seven AB, Barros-Alvarez X, de Lapeyriere M, Papasergi-Scott MM, Robertson MJ, Zhang C, Nwokonko RM, Gao Y, Meyerowitz JG, Rocher JP, Schelshorn D, Kobilka BK, Mathiesen JM, Skiniotis G. G-protein activation by a metabotropic glutamate receptor. Nature. 2021 Jun 30. pii: 10.1038/s41586-021-03680-3. doi:, 10.1038/s41586-021-03680-3. PMID:34194039 doi:http://dx.doi.org/10.1038/s41586-021-03680-3
- ↑ 4.0 4.1 4.2 4.3 Lin S, Han S, Cai X, Tan Q, Zhou K, Wang D, Wang X, Du J, Yi C, Chu X, Dai A, Zhou Y, Chen Y, Zhou Y, Liu H, Liu J, Yang D, Wang MW, Zhao Q, Wu B. Structures of Gi-bound metabotropic glutamate receptors mGlu2 and mGlu4. Nature. 2021 Jun;594(7864):583-588. doi: 10.1038/s41586-021-03495-2. Epub 2021, Jun 16. PMID:34135510 doi:http://dx.doi.org/10.1038/s41586-021-03495-2
- ↑ 5.0 5.1 5.2 5.3 5.4 Crupi R, Impellizzeri D, Cuzzocrea S. Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front Mol Neurosci. 2019 Feb 8;12:20. doi: 10.3389/fnmol.2019.00020. eCollection , 2019. PMID:30800054 doi:http://dx.doi.org/10.3389/fnmol.2019.00020
- ↑ Bordi F, Ugolini A. Group I metabotropic glutamate receptors: implications for brain diseases. Prog Neurobiol. 1999 Sep;59(1):55-79. doi: 10.1016/s0301-0082(98)00095-1. PMID:10416961 doi:http://dx.doi.org/10.1016/s0301-0082(98)00095-1
- ↑ Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009 Jan;30(1):25-31. doi: 10.1016/j.tips.2008.10.006. Epub, 2008 Dec 6. PMID:19058862 doi:http://dx.doi.org/10.1016/j.tips.2008.10.006
Student Contributors
- Courtney Vennekotter
- Cade Chezem