We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1715
From Proteopedia
(Difference between revisions)
| Line 31: | Line 31: | ||
|} | |} | ||
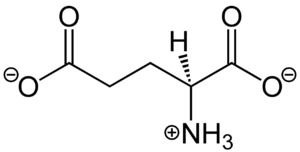
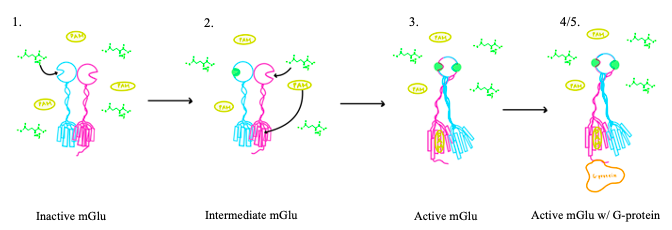
| - | [[Image:Glutamate.png|300 px|right|thumb|Figure 1. Structure of glutamate under physiological conditions (pH 7.4).]] To activate the mGlu transformation, glutamate acts as the protein's main agonist. Glutamate is an acidic, polar amino acid (Figure 1). This agonist binds to the extracellular portion of the glutamate receptor causing the transmembrane spanning region of the homodimer to change conformationally. This change allows for the mGlu to bind to a G-protein which initiates a signaling cascade within the cell that can ultimately lead to the modification of other proteins and a difference in the synapse’s excitability<ref name="Seven">PMID:34194039</ref>. In mGlu, the binding affinity of glutamate is also controlled by the binding of either a positive (PAM) or negative (NAM) allosteric modulator to a binding pocket within the seven TMD<ref name="Lin">PMID:34135510</ref>. | + | [[Image:Glutamate.png|300 px|right|thumb|Figure 1. Structure of glutamate under physiological conditions (pH 7.4).]] To activate the mGlu transformation, glutamate acts as the protein's main agonist. Glutamate is an acidic, polar amino acid (Figure 1). This agonist binds to the extracellular portion of the glutamate receptor causing the transmembrane spanning region of the homodimer to change conformationally. This change allows for the mGlu to bind to a G-protein. This binding allows for the activation of a G protein which initiates a signaling cascade within the cell that can ultimately lead to the modification of other proteins and a difference in the synapse’s excitability<ref name="Seven">PMID:34194039</ref>. In mGlu, the binding affinity of glutamate is also controlled by the binding of either a positive (PAM) or negative (NAM) allosteric modulator to a binding pocket within the seven TMD<ref name="Lin">PMID:34135510</ref>. |
== Structural Highlights == | == Structural Highlights == | ||
mGlu receptors are dimeric proteins consisting of an <scene name='90/904320/Inactive_mglu2/10'>alpha and beta chain</scene>. While a heterodimer of different mGlu subtypes can form, only homodimeric receptors can become active<ref name="Seven">PMID:34194039</ref>. Both the alpha and beta chains are comprised of <scene name='90/904320/Mglu2_domains/7'>3 domains</scene>: the venus fly trap (VFT), cysteine rich domain (CRD), and the transmembrane domain (TMD). | mGlu receptors are dimeric proteins consisting of an <scene name='90/904320/Inactive_mglu2/10'>alpha and beta chain</scene>. While a heterodimer of different mGlu subtypes can form, only homodimeric receptors can become active<ref name="Seven">PMID:34194039</ref>. Both the alpha and beta chains are comprised of <scene name='90/904320/Mglu2_domains/7'>3 domains</scene>: the venus fly trap (VFT), cysteine rich domain (CRD), and the transmembrane domain (TMD). | ||
| Line 54: | Line 54: | ||
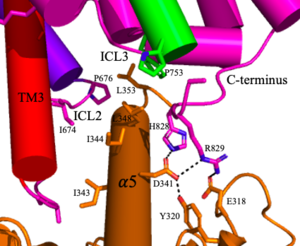
'''4.''' The crossover of the helices from the alpha and beta chains allows for intracellular loop 2 (ICL2) and the C-terminus to be properly ordered to interact with a single G protein<ref name="Seven">PMID:34194039</ref>. While hydrogen bonding is present between the C-terminus and alpha helix 5 of the G-protein, this coupling is primarily driven by the hydrophobic interactions in the interface with the ɑ5 helix of the G protein<ref name="Seven">PMID:34194039</ref>(Figure 4). This <scene name='90/904320/Active_mglu/5'>mGlu/G-protein coupling</scene> can only occur in the presence of a <scene name='90/904320/Pam/6'>PAM</scene> as the pocket in which the coupling occurs would be completely closed in its absence<ref name="Seven">PMID:34194039</ref>. | '''4.''' The crossover of the helices from the alpha and beta chains allows for intracellular loop 2 (ICL2) and the C-terminus to be properly ordered to interact with a single G protein<ref name="Seven">PMID:34194039</ref>. While hydrogen bonding is present between the C-terminus and alpha helix 5 of the G-protein, this coupling is primarily driven by the hydrophobic interactions in the interface with the ɑ5 helix of the G protein<ref name="Seven">PMID:34194039</ref>(Figure 4). This <scene name='90/904320/Active_mglu/5'>mGlu/G-protein coupling</scene> can only occur in the presence of a <scene name='90/904320/Pam/6'>PAM</scene> as the pocket in which the coupling occurs would be completely closed in its absence<ref name="Seven">PMID:34194039</ref>. | ||
| - | '''5.''' Upon binding, the G protein can become active through the receptor catalyzed reaction of GDP to GTP on the alpha subunit of the G protein. Depending on the type of mGlu present, this activation causes different signaling cascades to occur within the cell <ref name="Lin">PMID:34135510</ref>. These cascades are necessary for cellular function as they | + | '''5.''' Upon binding, the G protein can become active through the receptor catalyzed reaction of GDP to GTP on the alpha subunit of the G protein. Depending on the type of mGlu present, this activation causes different signaling cascades to occur within the cell <ref name="Lin">PMID:34135510</ref>. These cascades are necessary for cellular function as they can play primary roles in regulating metabolic molecules, ion channels, transporter molecules, and several other parts of the cell; if these proteins are mutated, various diseases can occur<ref name="Crupi">PMID:30800054</ref>. |
== Clinical Relevance == | == Clinical Relevance == | ||
Revision as of 02:33, 19 April 2022
Metabotropic Glutamate Receptor
| |||||||||||
Student Contributors
- Courtney Vennekotter
- Cade Chezem