We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1717
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<StructureSection load='' size='350' side='right' caption='Structure of Closed Vitamin K Epoxide Reductase (PDB entry [[6wv3]])' scene='90/904321/Closedconformation/2'> | <StructureSection load='' size='350' side='right' caption='Structure of Closed Vitamin K Epoxide Reductase (PDB entry [[6wv3]])' scene='90/904321/Closedconformation/2'> | ||
| - | |||
| - | |||
== Introduction == | == Introduction == | ||
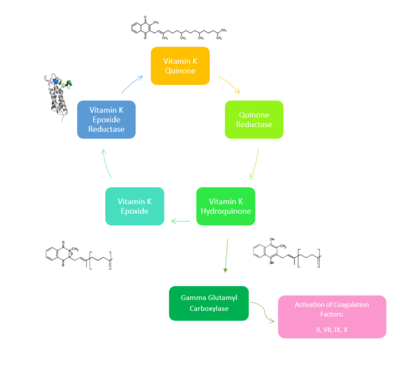
| - | [[Image:NewVitaminKCycle.PNG|400px|right|thumb|'''Figure | + | [[Image:NewVitaminKCycle.PNG|400px|right|thumb|'''Figure 1. Overview of Vitamin K Cycle''': The cycle begins with [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K Quinone]. Vitamin K Quinone is reduced by enzyme Quinone Reductase. This leaves Vitamin K Hydroquinone which can either lead to [https://en.wikipedia.org/wiki/Gamma-glutamyl_carboxylase Gamma Carboxylase]activity that will activate Blood Coagulation Factors II, VII, IX, and X. After this, Vitamin K Epoxide is left over. Vitamin K Epoxide is reduced by the enzyme Vitamin K Epoxide Reductase to reform Vitamin K Quinone. ]] |
<scene name='90/904321/Vitamin_k_epoxide_reductase/1'>Vitamin K Epoxide Reductase</scene> | <scene name='90/904321/Vitamin_k_epoxide_reductase/1'>Vitamin K Epoxide Reductase</scene> | ||
[https://en.wikipedia.org/wiki/Vitamin_K_epoxide_reductase VKOR WIKI](VKOR) is an endoplasmic membrane enzyme that generates the active form of Vitamin K to support blood coagulation. VKOR homologs are integral membrane thiol oxidoreductases [https://en.wikipedia.org/wiki/Thiol_oxidoreductase Thiol OxidoReductase] due to the function of VKOR being dependent on thiol residues and disulfide bonding. The Vitamin K Cycle and the VKOR enzyme specifically are common drug targets for thromboembolic diseases. This is because, as pictured, the vitamin K cycle is required to activate blood coagulant factors [https://en.wikipedia.org/wiki/Thrombin II], [https://en.wikipedia.org/wiki/Coagulation_factor_VII VII], [https://en.wikipedia.org/wiki/Factor_IX IX], and [https://en.wikipedia.org/wiki/Factor_X#:~:text=Factor%20X%2C%20also%20known%20by,vitamin%20K%20for%20its%20synthesis. X]. Coagulant factor activation promotes blood clotting, which in high amounts can be dangerous and cause thromboembolic diseases such as stroke, deep vein thrombosis, and/or pulmonary embolism. Vitamin K Epoxide Reductase is found and primarily synthesized in the liver. It is embedded in the membrane known as the endoplasmic reticulum. | [https://en.wikipedia.org/wiki/Vitamin_K_epoxide_reductase VKOR WIKI](VKOR) is an endoplasmic membrane enzyme that generates the active form of Vitamin K to support blood coagulation. VKOR homologs are integral membrane thiol oxidoreductases [https://en.wikipedia.org/wiki/Thiol_oxidoreductase Thiol OxidoReductase] due to the function of VKOR being dependent on thiol residues and disulfide bonding. The Vitamin K Cycle and the VKOR enzyme specifically are common drug targets for thromboembolic diseases. This is because, as pictured, the vitamin K cycle is required to activate blood coagulant factors [https://en.wikipedia.org/wiki/Thrombin II], [https://en.wikipedia.org/wiki/Coagulation_factor_VII VII], [https://en.wikipedia.org/wiki/Factor_IX IX], and [https://en.wikipedia.org/wiki/Factor_X#:~:text=Factor%20X%2C%20also%20known%20by,vitamin%20K%20for%20its%20synthesis. X]. Coagulant factor activation promotes blood clotting, which in high amounts can be dangerous and cause thromboembolic diseases such as stroke, deep vein thrombosis, and/or pulmonary embolism. Vitamin K Epoxide Reductase is found and primarily synthesized in the liver. It is embedded in the membrane known as the endoplasmic reticulum. | ||
| Line 27: | Line 25: | ||
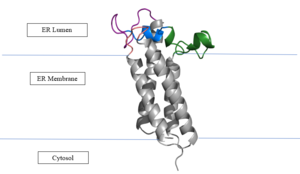
| - | [[Image:VKORmembrane.png|300px|left|thumb|Figure | + | [[Image:VKORmembrane.png|300px|left|thumb|Figure 2. Orientation in Endoplasmic Reticulum: The cap region is partially oriented in the ER Lumen, however the active site remains within the ER membrane. The Beta Hairpin, Loop 3-4, Cap Loop are all in the ER Lumen. The Anchor is partially within the ER lumen, and partially embedded in the ER membrane. The anchor is what attaches the cap domain and stabilizes it, which allows the cap domain to cover the active site. ]] |
The reaction catalyzed by VKOR is a redox reaction. ''Vitamin K Epoxide => Vitamin K Quinone'' Vitamin K Epoxide is reduced by transferring two electrons through a disulfide bond. These disulfide bonds come from the conserved cysteines. This redox reaction that is catalyzed by VKOR produces Vitamin K Quinone. | The reaction catalyzed by VKOR is a redox reaction. ''Vitamin K Epoxide => Vitamin K Quinone'' Vitamin K Epoxide is reduced by transferring two electrons through a disulfide bond. These disulfide bonds come from the conserved cysteines. This redox reaction that is catalyzed by VKOR produces Vitamin K Quinone. | ||
| Line 47: | Line 45: | ||
== Vitamin K Epoxide == | == Vitamin K Epoxide == | ||
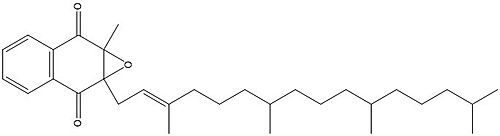
| - | [[Image:Vitamin K epoxide.jpg|500 px|right|thumb|Figure | + | [[Image:Vitamin K epoxide.jpg|500 px|right|thumb|Figure 3. Vitamin K Epoxide structure]] |
As mentioned above, Vitamin K epoxide is a part of the Vitamin K cycle and required for blood coagulation. In the cycle, VKOR reduces Vitamin K epoxide to Vitamin K Quinone, or the active form of Vitamin K. In this conversion, VKOR donates electrons to Vitamin K epoxide from the S-H of the active pair of cysteines, C132-C135. The mediated cysteine pair, C43-C51, has to be reduced for the transfer of electrons to the substrate to occur. | As mentioned above, Vitamin K epoxide is a part of the Vitamin K cycle and required for blood coagulation. In the cycle, VKOR reduces Vitamin K epoxide to Vitamin K Quinone, or the active form of Vitamin K. In this conversion, VKOR donates electrons to Vitamin K epoxide from the S-H of the active pair of cysteines, C132-C135. The mediated cysteine pair, C43-C51, has to be reduced for the transfer of electrons to the substrate to occur. | ||
| Line 53: | Line 51: | ||
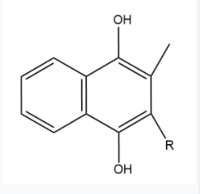
Two other notable structures are Vitamin K Quinone (Fig. 5) and Vitamin K Hydroquinone (Fig. 6). Vitamin K Quinone is the product that is released after the reaction with Vitamin K Epoxide and VKOR. (Fig. 2) | Two other notable structures are Vitamin K Quinone (Fig. 5) and Vitamin K Hydroquinone (Fig. 6). Vitamin K Quinone is the product that is released after the reaction with Vitamin K Epoxide and VKOR. (Fig. 2) | ||
| - | [[Image:Vitaminkquinone.PNG|200 px|left|thumb|Figure | + | [[Image:Vitaminkquinone.PNG|200 px|left|thumb|Figure 4. Vitamin K Quinone structure]] [[Image:Vitaminkhydroquinone.PNG|200 px|right|thumb|Figure 5. Vitamin K Hydroquinone structure]] |
| Line 65: | Line 63: | ||
== Warfarin == | == Warfarin == | ||
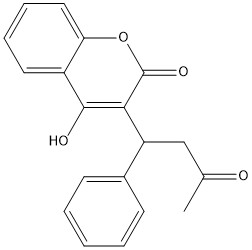
[https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common [https://en.wikipedia.org/wiki/Vitamin_K_antagonist Vitamin K antagonist (VKA)]. Warfarin is a competitive inhibitor, taking the place of Vitamin K Epoxide (VKO) in the active site of Vitamin K Epoxide Reductase (VKOR). When warfarin binds in the active site, it causes VKOR to go into the closed conformation. | [https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common [https://en.wikipedia.org/wiki/Vitamin_K_antagonist Vitamin K antagonist (VKA)]. Warfarin is a competitive inhibitor, taking the place of Vitamin K Epoxide (VKO) in the active site of Vitamin K Epoxide Reductase (VKOR). When warfarin binds in the active site, it causes VKOR to go into the closed conformation. | ||
| - | [[Image:warfarin.jpg|400 px|right|thumb|Figure | + | [[Image:warfarin.jpg|400 px|right|thumb|Figure 6. Warfarin structure]] |
=== Binding === | === Binding === | ||
Revision as of 02:58, 19 April 2022
| |||||||||||