We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1703
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
===Overall Structure=== | ===Overall Structure=== | ||
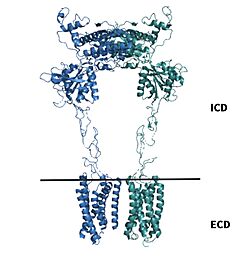
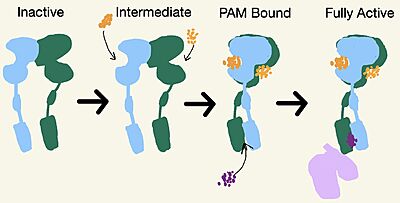
| - | [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy Cryo-EM] studies of mGlu2 have yielded adequate structural maps of mGlu2 in various activation states. These maps provided clearer understanding of the conformational changes between the inactive and active states of mGlu2<ref name="Lin" />. The conformational changes allow mGlu2 to move from an inactive <scene name='90/904307/Inactive_to_active_morph/1'>open to a closed</scene> active conformation. The overall <scene name='90/ | + | [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy Cryo-EM] studies of mGlu2 have yielded adequate structural maps of mGlu2 in various activation states. These maps provided clearer understanding of the conformational changes between the inactive and active states of mGlu2<ref name="Lin" />. The conformational changes allow mGlu2 to move from an inactive <scene name='90/904307/Inactive_to_active_morph/1'>open to a closed</scene> active conformation. The overall <scene name='90/904308/Inactive_structure/2'>structure</scene> of the mGlu2 is composed of 3 main parts. First, a ligand binding <scene name='90/904307/Better_inactive_structure/3'>Venus Fly Trap Domain(VFT)</scene>, that binds two glutamates which are the agonists. This is followed by a <scene name='90/904307/Better_inactive_structure/2'>Cysteine Rich Domain(CRD)</scene> that links the VFT to the TMD. The CRD is helpful in relaying signals for conformational changes in the TMD induced by agonist binding in the VFT<ref name="Lin" />. The VFT and CRD are located in the intracellular domain(ICD), while the TMD is located in the ECD (Figure 2). Finally the <scene name='90/904307/Better_inactive_structure/4'>TMD</scene> contains 7 α-helices (7TM) on both the α and β chains. The TMD aids in the binding of the G-protein. |
[[Image:Domains of mGlu2.jpg|250 px|right|thumb|'''Figure 2.'''Shown above the line is the intracellular region containing the VFT and CRD. Shown below the line is the extracellular region containing the TMD of mGlu2.]] | [[Image:Domains of mGlu2.jpg|250 px|right|thumb|'''Figure 2.'''Shown above the line is the intracellular region containing the VFT and CRD. Shown below the line is the extracellular region containing the TMD of mGlu2.]] | ||
Revision as of 04:38, 19 April 2022
Contents |
Metabotropic Glutamate Receptor 2
| |||||||||||
3D Structures
7mtq, mGlu2 inactive
7mtr, mGlu2 PAM bound
7mts, mGlu2 active
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 Lin S, Han S, Cai X, Tan Q, Zhou K, Wang D, Wang X, Du J, Yi C, Chu X, Dai A, Zhou Y, Chen Y, Zhou Y, Liu H, Liu J, Yang D, Wang MW, Zhao Q, Wu B. Structures of Gi-bound metabotropic glutamate receptors mGlu2 and mGlu4. Nature. 2021 Jun;594(7864):583-588. doi: 10.1038/s41586-021-03495-2. Epub 2021, Jun 16. PMID:34135510 doi:http://dx.doi.org/10.1038/s41586-021-03495-2
- ↑ 2.0 2.1 2.2 Seven, Alpay B., et al. “G-Protein Activation by a Metabotropic Glutamate Receptor.” Nature News, Nature Publishing Group, 30 June 2021, https://www.nature.com/articles/s1586-021-03680-3
- ↑ Du, Juan, et al. “Structures of Human mglu2 and mglu7 Homo- and Heterodimers.” Nature News, Nature Publishing Group, 16 June 2021, https://www.nature.com/articles/s41586-021-03641-w.>
- ↑ 4.0 4.1 “Metabotropic Glutamate Receptor.” Wikipedia, Wikimedia Foundation, 27 Mar. 2022, https://en.wikipedia.org/wiki/Metabotropic_glutamate_receptor
- ↑ 5.0 5.1 \“Schizophrenia.” National Institute of Mental Health, U.S. Department of Health and Human Services, https://www.nimh.nih.gov/health/topics/schizophrenia
- ↑ 6.0 6.1 Ellaithy A, Younkin J, Gonzalez-Maeso J, Logothetis DE. Positive allosteric modulators of metabotropic glutamate 2 receptors in schizophrenia treatment. Trends Neurosci. 2015 Aug;38(8):506-16. doi: 10.1016/j.tins.2015.06.002. Epub, 2015 Jul 4. PMID:26148747 doi:http://dx.doi.org/10.1016/j.tins.2015.06.002
- ↑ 7.0 7.1 7.2 7.3 Muguruza C, Meana JJ, Callado LF. Group II Metabotropic Glutamate Receptors as Targets for Novel Antipsychotic Drugs. Front Pharmacol. 2016 May 20;7:130. doi: 10.3389/fphar.2016.00130. eCollection, 2016. PMID:27242534 doi:http://dx.doi.org/10.3389/fphar.2016.00130
Student Contributors
Frannie Brewer Ashley Wilkinson