We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1715
From Proteopedia
(Difference between revisions)
| Line 37: | Line 37: | ||
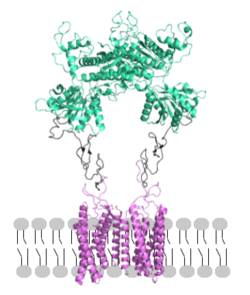
[[Image: Orientation_2.png|250px|left|thumb|Figure 2. Proper orientation of mGlu about the cell membrane.]] | [[Image: Orientation_2.png|250px|left|thumb|Figure 2. Proper orientation of mGlu about the cell membrane.]] | ||
==== Domains ==== | ==== Domains ==== | ||
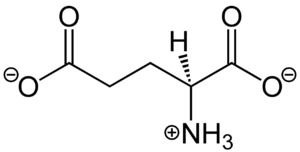
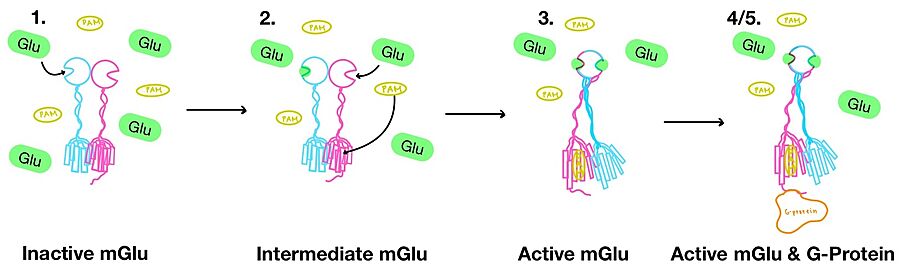
| - | <scene name='90/904320/Mglu2_domains_vft/3'>VFT</scene>: The extracellular location in which the two glutamate agonists bind is known as the VFT. This domain includes a disulfide bond between C121 of the alpha and beta chains. This <scene name='90/904320/Inactive_mglu/8'>inactive dimer disulfide bond</scene> is shifted down and undergoes an <scene name='90/904319/Cys_active/1'>upward movement</scene> upon glutamate binding which stabilizes the <scene name='90/ | + | <scene name='90/904320/Mglu2_domains_vft/3'>VFT</scene>: The extracellular location in which the two glutamate agonists bind is known as the VFT. This domain includes a disulfide bond between C121 of the alpha and beta chains. This <scene name='90/904320/Inactive_mglu/8'>inactive dimer disulfide bond</scene> is shifted down and undergoes an <scene name='90/904319/Cys_active/1'>upward movement</scene> upon glutamate binding which stabilizes the <scene name='90/904320/Active_site_interactions/4'>active site</scene> (Figure 2) Glutamate binds within the <scene name='90/904320/Active_site_interactions/3'>binding pocket</scene> through intermolecular forces, specifically hydrogen bonding, with R57, S143, S145, T168, and K377 of the VFT. This binding initiates a closed VFT conformation. |
<scene name='90/904320/Mglu2_domains_crd/3'>CRD</scene>: The portion of the protomer that connects the VFT with the TMD is known as the CRD. Many <scene name='90/904320/Crd_cysteine/3'>disulfide bonds</scene> are located in this region between cysteines. As the connecting segment of the protein, it is critical in transmitting the conformational change caused by the binding of glutamate to the TMD. The change resulting from the binding of glutamate in the VFT brings the cysteine-rich domains pf the alpha and beta chain together to alter the configuration of the the seven TMD helices through its interaction with the VFT extracellular loop 2 (ECL2) <ref name="Seven">PMID:34194039</ref>. This <scene name='90/904320/Active_helices/11'>ECL2 conformational change</scene> is mediated through interactions with amino acids at the apex of the CRD (e.g. I <ref name="Seven">PMID:34194039</ref>. | <scene name='90/904320/Mglu2_domains_crd/3'>CRD</scene>: The portion of the protomer that connects the VFT with the TMD is known as the CRD. Many <scene name='90/904320/Crd_cysteine/3'>disulfide bonds</scene> are located in this region between cysteines. As the connecting segment of the protein, it is critical in transmitting the conformational change caused by the binding of glutamate to the TMD. The change resulting from the binding of glutamate in the VFT brings the cysteine-rich domains pf the alpha and beta chain together to alter the configuration of the the seven TMD helices through its interaction with the VFT extracellular loop 2 (ECL2) <ref name="Seven">PMID:34194039</ref>. This <scene name='90/904320/Active_helices/11'>ECL2 conformational change</scene> is mediated through interactions with amino acids at the apex of the CRD (e.g. I <ref name="Seven">PMID:34194039</ref>. | ||
| Line 50: | Line 50: | ||
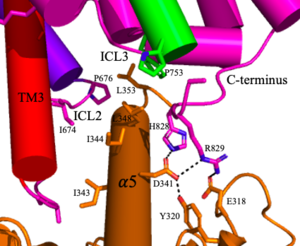
[[Image:Screen Shot 2022-04-18 at 10.20.26 PM.png|300 px|right|thumb|Figure 4. The interaction between an active mGlu (magenta/lime/purple/crimson) and a G-protein (orange). Hydrogen bonds are shown through black dashes]] | [[Image:Screen Shot 2022-04-18 at 10.20.26 PM.png|300 px|right|thumb|Figure 4. The interaction between an active mGlu (magenta/lime/purple/crimson) and a G-protein (orange). Hydrogen bonds are shown through black dashes]] | ||
| - | '''3.''' A second glutamate then binds to the other <scene name='90/904320/Active_site_interactions/ | + | '''3.''' A second glutamate then binds to the other <scene name='90/904320/Active_site_interactions/4'>binding pocket</scene> of the VFT. Mediated by L639, F643, N735, W773, and F776, a <scene name='90/904320/Pam/5'>positive allosteric modulator</scene> (PAM) also binds within the seven TMD helices of the alpha chain <ref name="Seven">PMID:34194039</ref>. This closed conformation of the VFT now has an inter-lobe angle of 25° is considered to be in the <scene name='90/904320/Active_mglu/6'>active conformation</scene><ref name="Seven">PMID:34194039</ref>. The binding of these ligands allows the CRDs to compact and come together. This transformation causes the TMD to form a separate, active asymmetric conformation with a <scene name='90/904319/Active_helices/23'>TM6-TM6 interface</scene> between the chains<ref name="Seven">PMID:34194039</ref>. |
'''4.''' The crossover of the helices from the alpha and beta chains allows for intracellular loop 2 (ICL2) and the C-terminus to be properly ordered to interact with a single G protein<ref name="Seven">PMID:34194039</ref>. While hydrogen bonding is present between the C-terminus and alpha helix 5 of the G-protein, this coupling is primarily driven by the hydrophobic interactions in the interface with the ɑ5 helix of the G protein<ref name="Seven">PMID:34194039</ref>(Figure 4). This <scene name='90/904320/Active_mglu/5'>mGlu/G-protein coupling</scene> can only occur in the presence of a <scene name='90/904320/Pam/6'>PAM</scene> as the pocket in which the coupling occurs would be completely closed in its absence<ref name="Seven">PMID:34194039</ref>. | '''4.''' The crossover of the helices from the alpha and beta chains allows for intracellular loop 2 (ICL2) and the C-terminus to be properly ordered to interact with a single G protein<ref name="Seven">PMID:34194039</ref>. While hydrogen bonding is present between the C-terminus and alpha helix 5 of the G-protein, this coupling is primarily driven by the hydrophobic interactions in the interface with the ɑ5 helix of the G protein<ref name="Seven">PMID:34194039</ref>(Figure 4). This <scene name='90/904320/Active_mglu/5'>mGlu/G-protein coupling</scene> can only occur in the presence of a <scene name='90/904320/Pam/6'>PAM</scene> as the pocket in which the coupling occurs would be completely closed in its absence<ref name="Seven">PMID:34194039</ref>. | ||
Revision as of 04:44, 19 April 2022
Metabotropic Glutamate Receptor
| |||||||||||
Student Contributors
- Courtney Vennekotter
- Cade Chezem