We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1715
From Proteopedia
(Difference between revisions)
| Line 41: | Line 41: | ||
<scene name='90/904320/Mglu2_domains_crd/7'>CRD</scene>: The portion of the protomer that connects the VFT with the TMD is known as the CRD. Many <scene name='90/904320/Crd_cysteine/3'>disulfide bonds</scene> are located in this region between cysteines. As the connecting segment of the protein, it is critical in transmitting the conformational change caused by the binding of glutamate to the TMD. The change resulting from the binding of glutamate in the VFT brings the cysteine-rich domains pf the alpha and beta chain together to alter the configuration of the the seven TMD helices through its interaction with the VFT extracellular loop 2 (ECL2) <ref name="Seven">PMID:34194039</ref>. This <scene name='90/904320/Active_helices/13'>ECL2 conformational change</scene> is mediated through interactions with amino acids at the apex of the CRD (e.g. I <ref name="Seven">PMID:34194039</ref>. | <scene name='90/904320/Mglu2_domains_crd/7'>CRD</scene>: The portion of the protomer that connects the VFT with the TMD is known as the CRD. Many <scene name='90/904320/Crd_cysteine/3'>disulfide bonds</scene> are located in this region between cysteines. As the connecting segment of the protein, it is critical in transmitting the conformational change caused by the binding of glutamate to the TMD. The change resulting from the binding of glutamate in the VFT brings the cysteine-rich domains pf the alpha and beta chain together to alter the configuration of the the seven TMD helices through its interaction with the VFT extracellular loop 2 (ECL2) <ref name="Seven">PMID:34194039</ref>. This <scene name='90/904320/Active_helices/13'>ECL2 conformational change</scene> is mediated through interactions with amino acids at the apex of the CRD (e.g. I <ref name="Seven">PMID:34194039</ref>. | ||
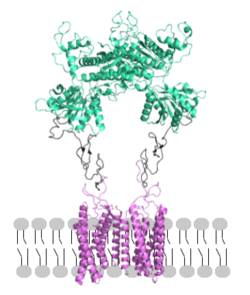
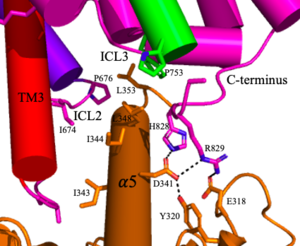
| - | <scene name='90/904320/Mglu2_domains_tmd/5'>TMD</scene>: The TMD consists of <scene name='90/904319/Inactive_tmd/9'>seven transmembrane helices</scene> that are responsible for G-protein interactions and are able to transmit the signal from ligand binding across a membrane. In the <scene name='90/904320/Inactive_mglu2_first_picture/5'>inactive form</scene>, the asymmetric conformation of the helices is mediated by the hydrophobicity of helix 3 and 4 <ref name="Seven">PMID:34194039</ref>. This allows for a <scene name='90/ | + | <scene name='90/904320/Mglu2_domains_tmd/5'>TMD</scene>: The TMD consists of <scene name='90/904319/Inactive_tmd/9'>seven transmembrane helices</scene> that are responsible for G-protein interactions and are able to transmit the signal from ligand binding across a membrane. In the <scene name='90/904320/Inactive_mglu2_first_picture/5'>inactive form</scene>, the asymmetric conformation of the helices is mediated by the hydrophobicity of helix 3 and 4 <ref name="Seven">PMID:34194039</ref>. This allows for a <scene name='90/904320/Inactive_tmd_interface/1'>TM3-TM4 interface</scene> to form between the monomers. Along with the interaction of the CRD with the ECL2 of the TMD, an allosteric modulator must bind within the transmembrane helices to allow for the conformation of the helices to be altered. This conformation allows for an <scene name='90/904319/Active_helices/23'>active dimer interface</scene> along helix 6 of both protomers <ref name="Lin">PMID:34135510</ref>. The stabilization of this conformation also enables G protein coupling with ICL2, ICL3, TM Helix 3 and the C terminus <ref name="Lin">PMID:34135510</ref> (Figure 4). |
== Conformational Changes == | == Conformational Changes == | ||
| Line 47: | Line 47: | ||
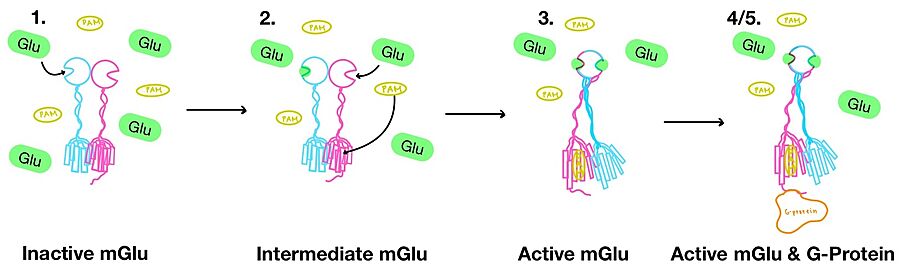
'''1.''' In its resting state, mGlu is in an <scene name='90/904320/Inactive_mglu/4'>inactive homodimeric form</scene>. In this conformation, the receptor is considered open with an inter-lobe angle of 44°<ref name="Seven">PMID:34194039</ref>. The structure has two free glutamate binding sites in the VFT, the CRDs are separated, and the TMD is not interacting with a G protein<ref name="Seven">PMID:34194039</ref>. | '''1.''' In its resting state, mGlu is in an <scene name='90/904320/Inactive_mglu/4'>inactive homodimeric form</scene>. In this conformation, the receptor is considered open with an inter-lobe angle of 44°<ref name="Seven">PMID:34194039</ref>. The structure has two free glutamate binding sites in the VFT, the CRDs are separated, and the TMD is not interacting with a G protein<ref name="Seven">PMID:34194039</ref>. | ||
| - | '''2.''' In the intermediate activation state, also known as the open-closed conformation, one glutamate is bound in one binding pocket of VFT. This single <scene name='90/904320/Mglu_binding/8'>glutamate bound state</scene> is still considered inactive as the receptor has not changed the conformations in the CRD and thus the TMD. With the same asymmetric transmembrane helices formation, a <scene name='90/ | + | '''2.''' In the intermediate activation state, also known as the open-closed conformation, one glutamate is bound in one binding pocket of VFT. This single <scene name='90/904320/Mglu_binding/8'>glutamate bound state</scene> is still considered inactive as the receptor has not changed the conformations in the CRD and thus the TMD. With the same asymmetric transmembrane helices formation, a <scene name='90/904320/Inactive_tmd_interface/1'>TM3-TM4 interface</scene> is still present and mGlu cannot interact with a G protein<ref name="Seven">PMID:34194039</ref>. |
[[Image:Screen Shot 2022-04-18 at 10.20.26 PM.png|300 px|right|thumb|Figure 4. The interaction between an active mGlu (magenta/lime/purple/crimson) and a G-protein (orange). Hydrogen bonds are shown through black dashes]] | [[Image:Screen Shot 2022-04-18 at 10.20.26 PM.png|300 px|right|thumb|Figure 4. The interaction between an active mGlu (magenta/lime/purple/crimson) and a G-protein (orange). Hydrogen bonds are shown through black dashes]] | ||
Revision as of 06:19, 19 April 2022
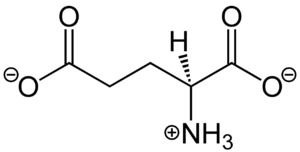
Metabotropic Glutamate Receptor
| |||||||||||
Student Contributors
- Courtney Vennekotter
- Cade Chezem