Sandbox Reserved 1716
From Proteopedia
| Line 60: | Line 60: | ||
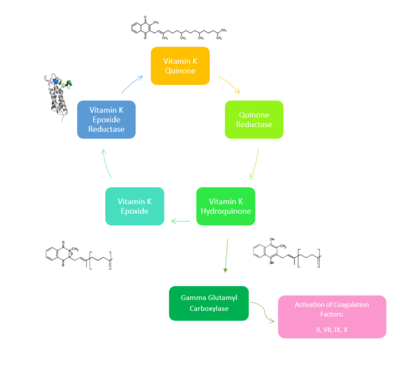
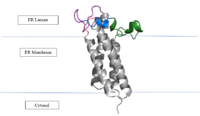
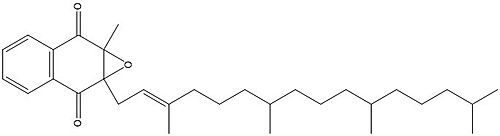
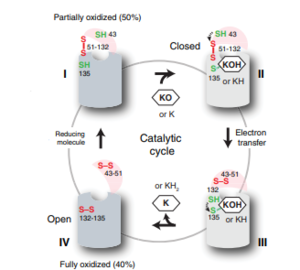
| - | VKOR is the second enzyme in the Vitamin K Cycle (Fig. 1), and has its own catalytic cycle as well. Step <scene name='90/904321/I/1'>I</scene> of this VKOR cycle begins in a partially oxidized open conformation. In this state, catalytic cysteines 51 and 132 form a disulfide bond. Cysteines 43 and 135 are considered "free" because they are not bound to anything in this state. Step <scene name='90/904321/Ii/2'>II</scene> is initiated when Vitamin K Epoxide binds to the active site. This binding induces a conformation change that "closes" the enzyme. In step II the cysteines remain in the same configuration, except Cys135 which forms a bond with the 3' hydroxyl group of Vitamin K Epoxide. In step <scene name='90/904321/Iii/1'>III</scene>, a bond between Cys43 and Cys51 causes an electron transfer to Cys132. In its reduced form Cys132 will attack Cys135, and the extra electrons are kicked to Vitamin K Epoxide. This opens the epoxide ring on Vitamin K Epoxide so that it may be reformed into Vitamin K Quinone. Vitamin K Quinone is released from Vitamin K Epoxide Reductase. This is step <scene name='90/904321/Iv/1'>IV</scene>, which is a fully oxidized open conformation of VKOR. This process is repeated over and over unless interrupted by inhibitors known as Vitamin K Antagonists or VKAs. | + | VKOR is the second enzyme in the Vitamin K Cycle (Fig. 1), and has its own catalytic cycle as well. Step <scene name='90/904321/I/1'>I</scene> of this VKOR cycle begins in a partially oxidized open conformation. In this state, catalytic cysteines 51 and 132 form a disulfide bond. Cysteines 43 and 135 are considered "free" because they are not bound to anything in this state. Step <scene name='90/904321/Ii/2'>II</scene> is initiated when Vitamin K Epoxide binds to the active site.<ref name="Goodstadt">PMID:15276181</ref> This binding induces a conformation change that "closes" the enzyme. In step II the cysteines remain in the same configuration, except Cys135 which forms a bond with the 3' hydroxyl group of Vitamin K Epoxide. In step <scene name='90/904321/Iii/1'>III</scene>, a bond between Cys43 and Cys51 causes an electron transfer to Cys132. In its reduced form Cys132 will attack Cys135, and the extra electrons are kicked to Vitamin K Epoxide. This opens the epoxide ring on Vitamin K Epoxide so that it may be reformed into Vitamin K Quinone. Vitamin K Quinone is released from Vitamin K Epoxide Reductase. This is step <scene name='90/904321/Iv/1'>IV</scene>, which is a fully oxidized open conformation of VKOR. This process is repeated over and over unless interrupted by inhibitors known as Vitamin K Antagonists or VKAs. |
| Line 101: | Line 101: | ||
| - | (DON’T INCLUDE) | ||
<ref name="Goodstadt">PMID:15276181</ref> Goodstadt, L., & Ponting, C. P. (2004). Vitamin K epoxide reductase: homology, active site and catalytic mechanism. ''Trends in biochemical sciences, 29''(6), 289–292. https://doi.org/10.1016/j.tibs.2004.04.004 | <ref name="Goodstadt">PMID:15276181</ref> Goodstadt, L., & Ponting, C. P. (2004). Vitamin K epoxide reductase: homology, active site and catalytic mechanism. ''Trends in biochemical sciences, 29''(6), 289–292. https://doi.org/10.1016/j.tibs.2004.04.004 | ||
Rishavy, M.A., Usubalieva, A., Hallgren, K.W., & Berkner, K.L. (2011). Novel insidht into the mechanism of the vitamin K oxidoreductas (VKOR): Electron relay through Cy43 and Cys51 reduces VKOR to allow vitamin K reduction and facilitation of vitamin K-dependent protein caroxylation. ''Journal of Biological Chemistry, 286''(9), 7267-7278. https://doi.org/10.1074/jbc.M110.172213 | Rishavy, M.A., Usubalieva, A., Hallgren, K.W., & Berkner, K.L. (2011). Novel insidht into the mechanism of the vitamin K oxidoreductas (VKOR): Electron relay through Cy43 and Cys51 reduces VKOR to allow vitamin K reduction and facilitation of vitamin K-dependent protein caroxylation. ''Journal of Biological Chemistry, 286''(9), 7267-7278. https://doi.org/10.1074/jbc.M110.172213 | ||
| - | <ref name= | + | <ref name=”Liu”>PMID:33154105</ref> Liu, S., Li, S., Shen, G., Sukumar, N., Krezel, A. M., & Li, W. (2021). Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. ''Science (New York, N.Y.), 371''(6524), eabc5667. https://doi.org/10.1126/science.abc5667 |
<ref name="Shen">PMID:33273012</ref> Shen, G., Cui, W., Cao, Q., Gao, M., Liu, H., Su, G., Gross, M. L., & Li, W. (2021). The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. ''The Journal of biological chemistry'', 296, 100145. https://doi.org/10.1074/jbc.RA120.015401 | <ref name="Shen">PMID:33273012</ref> Shen, G., Cui, W., Cao, Q., Gao, M., Liu, H., Su, G., Gross, M. L., & Li, W. (2021). The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. ''The Journal of biological chemistry'', 296, 100145. https://doi.org/10.1074/jbc.RA120.015401 | ||
Revision as of 14:12, 19 April 2022
Vitamin K Epoxide Reductase
| |||||||||||
References
- ↑ Shen G, Cui W, Cao Q, Gao M, Liu H, Su G, Gross ML, Li W. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. J Biol Chem. 2021 Jan-Jun;296:100145. doi: 10.1074/jbc.RA120.015401. Epub 2020, Dec 10. PMID:33273012 doi:http://dx.doi.org/10.1074/jbc.RA120.015401
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Goodstadt L, Ponting CP. Vitamin K epoxide reductase: homology, active site and catalytic mechanism. Trends Biochem Sci. 2004 Jun;29(6):289-92. doi: 10.1016/j.tibs.2004.04.004. PMID:15276181 doi:http://dx.doi.org/10.1016/j.tibs.2004.04.004
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ PMID:23034830<ref></ref>Warfarin is the most common medication for this treatment, acting as a blood thinner. Warfarin binding to VKOR prevents the triggering of coagulation factors that form blood clots.
[1] Goodstadt, L., & Ponting, C. P. (2004). Vitamin K epoxide reductase: homology, active site and catalytic mechanism. Trends in biochemical sciences, 29(6), 289–292. https://doi.org/10.1016/j.tibs.2004.04.004
Rishavy, M.A., Usubalieva, A., Hallgren, K.W., & Berkner, K.L. (2011). Novel insidht into the mechanism of the vitamin K oxidoreductas (VKOR): Electron relay through Cy43 and Cys51 reduces VKOR to allow vitamin K reduction and facilitation of vitamin K-dependent protein caroxylation. Journal of Biological Chemistry, 286(9), 7267-7278. https://doi.org/10.1074/jbc.M110.172213
[2] Liu, S., Li, S., Shen, G., Sukumar, N., Krezel, A. M., & Li, W. (2021). Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science (New York, N.Y.), 371(6524), eabc5667. https://doi.org/10.1126/science.abc5667
[3] Shen, G., Cui, W., Cao, Q., Gao, M., Liu, H., Su, G., Gross, M. L., & Li, W. (2021). The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. The Journal of biological chemistry, 296, 100145. https://doi.org/10.1074/jbc.RA120.015401
Silverman, R.B. (1981). Chemical model studies for the mechanism of vitamin K epoxide reductase. The Journal of American Chemistry Society, 103(19), 5939-5941. [4] [5] [6] [7] [8]