This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1715
From Proteopedia
(Difference between revisions)
| Line 31: | Line 31: | ||
|} | |} | ||
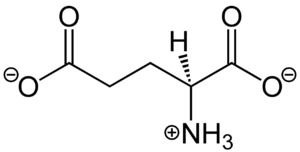
| - | [[Image:Glutamate.png|300 px|right|thumb|Figure 1. Structure of glutamate under physiological conditions (pH 7.4).]] To activate the mGlu transformation, glutamate acts as the protein's main [https://en.wikipedia.org/wiki/Agonist agonist]. [https://en.wikipedia.org/wiki/Glutamic_acid Glutamate] is an acidic, polar [https://en.wikipedia.org/wiki/Amino_acid amino acid] (Figure 1). This agonist binds to the extracellular portion of the glutamate receptor causing the transmembrane spanning region of the homodimer to change conformationally. This change allows for the mGlu to bind to a G-protein. This binding allows for the activation of a G protein which initiates a signaling cascade within the cell that can ultimately lead to a difference in the synapse’s excitability<ref name="Seven">PMID:34194039</ref>. In mGlu, the binding affinity of glutamate is also controlled by the binding of either a positive (PAM) or negative (NAM) [https://en.wikipedia.org/wiki/Allosteric_modulator allosteric modulator] to a [https://en.wikipedia.org/wiki/Binding_site binding site] within the seven TMD<ref name="Lin">PMID:34135510</ref>. | + | [[Image:Glutamate.png|300 px|right|thumb|Figure 1. Structure of glutamate under physiological conditions (pH 7.4).]] To activate the mGlu transformation, glutamate acts as the protein's main [https://en.wikipedia.org/wiki/Agonist agonist]. [https://en.wikipedia.org/wiki/Glutamic_acid Glutamate] is an acidic, polar [https://en.wikipedia.org/wiki/Amino_acid amino acid] (Figure 1). This agonist binds to the extracellular portion of the glutamate receptor causing the transmembrane spanning region of the homodimer to change conformationally. This change allows for the mGlu to bind to a G-protein. This binding allows for the activation of a G-protein which initiates a signaling cascade within the cell that can ultimately lead to a difference in the synapse’s excitability<ref name="Seven">PMID:34194039</ref>. In mGlu, the binding affinity of glutamate is also controlled by the binding of either a positive (PAM) or negative (NAM) [https://en.wikipedia.org/wiki/Allosteric_modulator allosteric modulator] to a [https://en.wikipedia.org/wiki/Binding_site binding site] within the seven TMD<ref name="Lin">PMID:34135510</ref>. |
== Structural Highlights == | == Structural Highlights == | ||
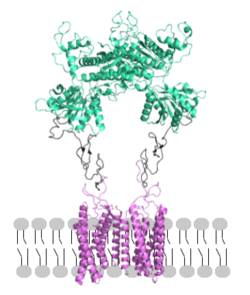
mGlu receptors are [https://en.wikipedia.org/wiki/Protein_dimer dimeric proteins] consisting of an <scene name='90/904320/Inactive_chains/3'>alpha and beta chain</scene><ref name="Seven">PMID:34194039</ref>. While a heterodimer of different mGlu subtypes can form, only homodimeric receptors can become active<ref name="Seven">PMID:34194039</ref>. Both the alpha and beta chains are comprised of <scene name='90/904320/Mglu2_domains/9'>three domains</scene>: the venus fly trap (VFT), cysteine rich domain (CRD), and the transmembrane domain (TMD)<ref name="Seven">PMID:34194039</ref>. | mGlu receptors are [https://en.wikipedia.org/wiki/Protein_dimer dimeric proteins] consisting of an <scene name='90/904320/Inactive_chains/3'>alpha and beta chain</scene><ref name="Seven">PMID:34194039</ref>. While a heterodimer of different mGlu subtypes can form, only homodimeric receptors can become active<ref name="Seven">PMID:34194039</ref>. Both the alpha and beta chains are comprised of <scene name='90/904320/Mglu2_domains/9'>three domains</scene>: the venus fly trap (VFT), cysteine rich domain (CRD), and the transmembrane domain (TMD)<ref name="Seven">PMID:34194039</ref>. | ||
| Line 41: | Line 41: | ||
<scene name='90/904320/Mglu2_domains_crd/7'>CRD</scene>: The portion of the protomer that connects the VFT with the TMD is known as the CRD. Many <scene name='90/904320/Crd_cysteine/3'>disulfide bonds</scene> are located in this region between cysteines. As the connecting segment of the protein, it is critical in transmitting the conformational change caused by the binding of glutamate in the VFT to the TMD<ref name="Seven">PMID:34194039</ref>. The change resulting from the binding of glutamate brings the cysteine-rich domains of the alpha and beta chain together to alter the configuration of the seven TMD helices through its interaction with the VFT extracellular loop 2 (ECL2) <ref name="Seven">PMID:34194039</ref>. This <scene name='90/904320/Active_helices/13'>ECL2 conformational change</scene> is mediated through interactions with amino acids at the apex of the CRD (e.g. I674, P676, and P753) <ref name="Seven">PMID:34194039</ref>. | <scene name='90/904320/Mglu2_domains_crd/7'>CRD</scene>: The portion of the protomer that connects the VFT with the TMD is known as the CRD. Many <scene name='90/904320/Crd_cysteine/3'>disulfide bonds</scene> are located in this region between cysteines. As the connecting segment of the protein, it is critical in transmitting the conformational change caused by the binding of glutamate in the VFT to the TMD<ref name="Seven">PMID:34194039</ref>. The change resulting from the binding of glutamate brings the cysteine-rich domains of the alpha and beta chain together to alter the configuration of the seven TMD helices through its interaction with the VFT extracellular loop 2 (ECL2) <ref name="Seven">PMID:34194039</ref>. This <scene name='90/904320/Active_helices/13'>ECL2 conformational change</scene> is mediated through interactions with amino acids at the apex of the CRD (e.g. I674, P676, and P753) <ref name="Seven">PMID:34194039</ref>. | ||
| - | <scene name='90/904320/Mglu2_domains_tmd/5'>TMD</scene>: The TMD consists of <scene name='90/904319/Inactive_tmd/9'>seven transmembrane helices</scene> that are responsible for G-protein interactions and are able to transmit the signal from ligand binding across a membrane<ref name="Niswender">PMID:20055706</ref>. In the <scene name='90/904320/Inactive_mglu2_first_picture/5'>inactive form</scene>, the asymmetric conformation of the helices is mediated by the hydrophobicity of helix 3 and 4 <ref name="Seven">PMID:34194039</ref>. This allows for a <scene name='90/904320/Inactive_tmd_interface/1'>TM3-TM4 interface</scene> to form between the monomers (Figure 3A). Along with the interaction of the CRD with the ECL2 of the TMD, an allosteric modulator must bind within the transmembrane helices to allow for the conformation of the helices to be altered<ref name="Seven">PMID:34194039</ref>. This conformation allows for an <scene name='90/904320/Active_helices/14'>active dimer interface</scene> along helix 6 of both protomers (Figure 3B)<ref name="Lin">PMID:34135510</ref>. The stabilization of this conformation also enables G protein coupling with ICL2, ICL3, TM Helix 3 and the C terminus <ref name="Lin">PMID:34135510</ref> (Figure 5). | + | <scene name='90/904320/Mglu2_domains_tmd/5'>TMD</scene>: The TMD consists of <scene name='90/904319/Inactive_tmd/9'>seven transmembrane helices</scene> that are responsible for G-protein interactions and are able to transmit the signal from ligand binding across a membrane<ref name="Niswender">PMID:20055706</ref>. In the <scene name='90/904320/Inactive_mglu2_first_picture/5'>inactive form</scene>, the asymmetric conformation of the helices is mediated by the hydrophobicity of helix 3 and 4 <ref name="Seven">PMID:34194039</ref>. This allows for a <scene name='90/904320/Inactive_tmd_interface/1'>TM3-TM4 interface</scene> to form between the monomers (Figure 3A). Along with the interaction of the CRD with the ECL2 of the TMD, an allosteric modulator must bind within the transmembrane helices to allow for the conformation of the helices to be altered<ref name="Seven">PMID:34194039</ref>. This conformation allows for an <scene name='90/904320/Active_helices/14'>active dimer interface</scene> along helix 6 of both protomers (Figure 3B)<ref name="Lin">PMID:34135510</ref>. The stabilization of this conformation also enables G-protein coupling with ICL2, ICL3, TM Helix 3 and the C terminus <ref name="Lin">PMID:34135510</ref> (Figure 5). |
[[Image:Screen Shot 2022-04-19 at 2.52.21 AM.png|500px|center|thumb|Figure 3. A) The inactive transmembrane helices conformation. B) The active transmembrane helices conformation.]] | [[Image:Screen Shot 2022-04-19 at 2.52.21 AM.png|500px|center|thumb|Figure 3. A) The inactive transmembrane helices conformation. B) The active transmembrane helices conformation.]] | ||
== Conformational Changes == | == Conformational Changes == | ||
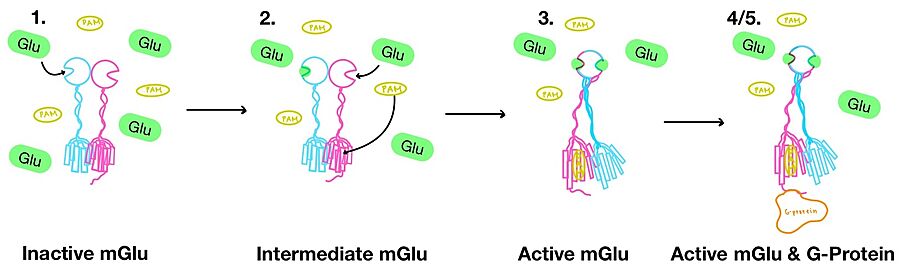
| - | '''1.''' (Figure 3-1) In its resting state, mGlu is in an <scene name='90/904320/Inactive_mglu2_first_picture/6'>inactive homodimeric form</scene>. In this conformation, the receptor is considered open with an inter-lobe angle of 44°<ref name="Seven">PMID:34194039</ref>. The structure has two free glutamate binding sites in the VFT, the CRDs are separated, and the TMD is not interacting with a G protein<ref name="Seven">PMID:34194039</ref>. | + | '''1.''' (Figure 3-1) In its resting state, mGlu is in an <scene name='90/904320/Inactive_mglu2_first_picture/6'>inactive homodimeric form</scene>. In this conformation, the receptor is considered open with an inter-lobe angle of 44°<ref name="Seven">PMID:34194039</ref>. The structure has two free glutamate binding sites in the VFT, the CRDs are separated, and the TMD is not interacting with a G-protein<ref name="Seven">PMID:34194039</ref>. |
| - | '''2.''' (Figure 3-2) In the intermediate activation state, also known as the open-closed conformation, one glutamate is bound in one binding pocket of VFT. This single <scene name='90/904320/Mglu_binding/9'>glutamate bound state</scene> is still considered inactive as the receptor has not changed the conformations in the CRD and thus the TMD. With the same asymmetric transmembrane helices formation, a <scene name='90/904320/Inactive_tmd_interface/1'>TM3-TM4 interface</scene> is still present and mGlu cannot interact with a G protein<ref name="Seven">PMID:34194039</ref>. | + | '''2.''' (Figure 3-2) In the intermediate activation state, also known as the open-closed conformation, one glutamate is bound in one binding pocket of VFT. This single <scene name='90/904320/Mglu_binding/9'>glutamate bound state</scene> is still considered inactive as the receptor has not changed the conformations in the CRD and thus the TMD. With the same asymmetric transmembrane helices formation, a <scene name='90/904320/Inactive_tmd_interface/1'>TM3-TM4 interface</scene> is still present and mGlu cannot interact with a G-protein<ref name="Seven">PMID:34194039</ref>. |
[[Image:Overview_mGlu_2.jpg|900 px|center|thumb|Figure 4. Illustration of mGlu's conformational change process.]] | [[Image:Overview_mGlu_2.jpg|900 px|center|thumb|Figure 4. Illustration of mGlu's conformational change process.]] | ||
| Line 55: | Line 55: | ||
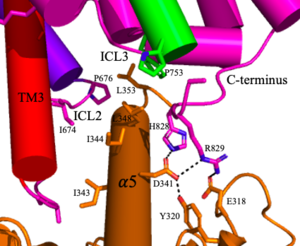
[[Image:Screen Shot 2022-04-18 at 10.20.26 PM.png|300 px|right|thumb|Figure 5. The interaction between an active mGlu (magenta/lime/purple/crimson) and a G-protein (orange). Hydrogen bonds are shown through black dashes]] | [[Image:Screen Shot 2022-04-18 at 10.20.26 PM.png|300 px|right|thumb|Figure 5. The interaction between an active mGlu (magenta/lime/purple/crimson) and a G-protein (orange). Hydrogen bonds are shown through black dashes]] | ||
| - | '''4.''' (Figure 3-4/5) The crossover of the helices from the alpha and beta chains allows for intracellular loop 2 (ICL2) and the C-terminus to be properly ordered to interact with a single G protein<ref name="Seven">PMID:34194039</ref>. While hydrogen bonding is present between the C-terminus and alpha helix 5 of the G-protein, this <scene name='90/904320/Active_mglu/9'>mGlu/G-protein coupling</scene> is primarily driven by the hydrophobic interactions in the interface with the ɑ5 helix of the G protein<ref name="Seven">PMID:34194039</ref>(Figure 5). This coupling can only occur in the presence of a <scene name='90/904320/Pam/8'>PAM</scene> as the pocket in which the coupling occurs would be completely closed in its absence<ref name="Seven">PMID:34194039</ref>. | + | '''4.''' (Figure 3-4/5) The crossover of the helices from the alpha and beta chains allows for intracellular loop 2 (ICL2) and the C-terminus to be properly ordered to interact with a single G-protein<ref name="Seven">PMID:34194039</ref>. While hydrogen bonding is present between the C-terminus and alpha helix 5 of the G-protein, this <scene name='90/904320/Active_mglu/9'>mGlu/G-protein coupling</scene> is primarily driven by the hydrophobic interactions in the interface with the ɑ5 helix of the G-protein<ref name="Seven">PMID:34194039</ref>(Figure 5). This coupling can only occur in the presence of a <scene name='90/904320/Pam/8'>PAM</scene> as the pocket in which the coupling occurs would be completely closed in its absence<ref name="Seven">PMID:34194039</ref>. |
| - | '''5.''' (Figure 3-4/5) Upon binding, the G protein can become active through the receptor catalyzed reaction of GDP to GTP on the alpha subunit of the G protein. Depending on the type of mGlu present, this activation causes different signaling cascades to occur within the cell <ref name="Lin">PMID:34135510</ref>. These cascades are necessary for cellular function as they can play primary roles in regulating metabolic molecules, ion channels, transporter molecules, and several other parts of the cell; if these proteins are mutated, various diseases can occur<ref name="Crupi">PMID:30800054</ref>. | + | '''5.''' (Figure 3-4/5) Upon binding, the G-protein can become active through the receptor catalyzed reaction of GDP to GTP on the alpha subunit of the G-protein. Depending on the type of mGlu present, this activation causes different signaling cascades to occur within the cell <ref name="Lin">PMID:34135510</ref>. These cascades are necessary for cellular function as they can play primary roles in regulating metabolic molecules, ion channels, transporter molecules, and several other parts of the cell; if these proteins are mutated, various diseases can occur<ref name="Crupi">PMID:30800054</ref>. |
== Clinical Relevance == | == Clinical Relevance == | ||
| Line 66: | Line 66: | ||
[[7mtr]], mGlu Active <br /> | [[7mtr]], mGlu Active <br /> | ||
[[7epb]], mGlu Active <br /> | [[7epb]], mGlu Active <br /> | ||
| - | [[7mts]], mGlu Active G Protein Bound <br /> | + | [[7mts]], mGlu Active G-Protein Bound <br /> |
== References == | == References == | ||
Revision as of 14:21, 19 April 2022
Metabotropic Glutamate Receptor
| |||||||||||
Student Contributors
- Courtney Vennekotter
- Cade Chezem