We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1716
From Proteopedia
(Difference between revisions)
| Line 50: | Line 50: | ||
==Catalytic Cycle== | ==Catalytic Cycle== | ||
| - | [[Image:VKORcycle.PNG|300px|right|thumb|'''Figure | + | [[Image:VKORcycle.PNG|300px|right|thumb|'''Figure 6: The catalytic cycle of Vitamin K Epoxide Reductase''' <ref name="Liu"/> ]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
===Step I === | ===Step I === | ||
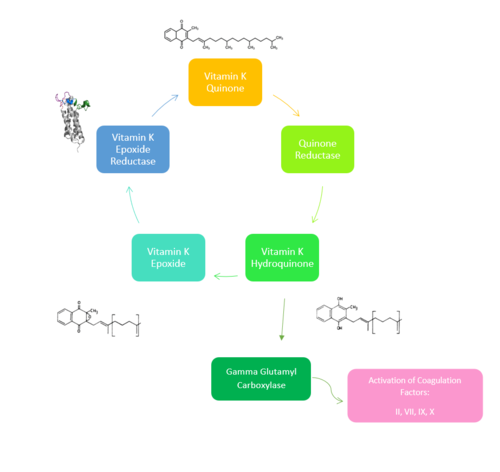
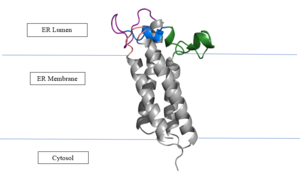
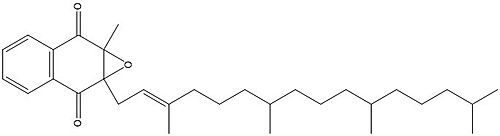
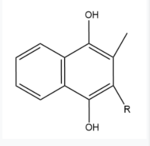
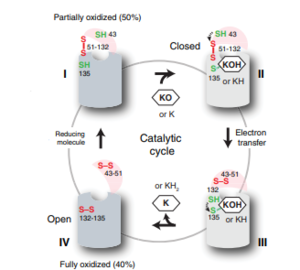
| - | <scene name='90/904321/I/1'>Step I</scene> of reforming Vitamin K Epoxide (Fig. 3) through the enzyme Vitamin K Reductase (VKOR) begins in a partially oxidized open conformation. In this state, catalytic cysteines 51 and 132 form a disulfide bond. Cysteines 43 and 135 are considered "free" because they are not bound to anything in this state. The <scene name='90/904321/I/2'>central binding pocket</scene> (highlighted in hot pink) is also empty because Vitamin K Epoxide has not bound yet. In order to get to the next step, Vitamin K epoxide will enter through the isoprenyl-chain tunnel.<ref name="Liu"/> | + | VKOR is the second enzyme in the Vitamin K Cycle (Fig. 1), and has its own catalytic cycle as well. <scene name='90/904321/I/1'>Step I</scene> of reforming Vitamin K Epoxide (Fig. 3) through the enzyme Vitamin K Reductase (VKOR) begins in a partially oxidized open conformation. In this state, catalytic cysteines 51 and 132 form a disulfide bond. Cysteines 43 and 135 are considered "free" because they are not bound to anything in this state. The <scene name='90/904321/I/2'>central binding pocket</scene> (highlighted in hot pink) is also empty because Vitamin K Epoxide has not bound yet. In order to get to the next step, Vitamin K epoxide will enter through the isoprenyl-chain tunnel.<ref name="Liu"/> |
===Step II=== | ===Step II=== | ||
| - | After Vitamin K Epoxide enters through the isoprenyl-chain tunnel, this begins Step II<scene name='90/904321/Ii/2'>Step II</scene>. Asn80 on TM2 and Tyr139 on TM4 <scene name='90/904322/Tyr_asn_binding_warfarin/2'>hydrogen bond</scene>. to Vitamin K Epoxide. When Vitamin K Epoxide binds, there is a shift in the bonds between the cap domain, beta hairpin, and anchor. <scene name='90/904321/Vkobound_cys/1'>Cys135</scene> also forms a disulfide bond with the 3' OH group on Vitamin K Epoxide. | + | After Vitamin K Epoxide enters through the isoprenyl-chain tunnel, this begins Step II<scene name='90/904321/Ii/2'>Step II</scene>. This binding induces a conformation change that "closes" the enzyme.Asn80 on TM2 and Tyr139 on TM4 <scene name='90/904322/Tyr_asn_binding_warfarin/2'>hydrogen bond</scene>. to Vitamin K Epoxide. When Vitamin K Epoxide binds, there is a shift in the bonds between the cap domain, beta hairpin, and anchor. <scene name='90/904321/Vkobound_cys/1'>Cys135</scene> also forms a disulfide bond with the 3' OH group on Vitamin K Epoxide. |
===Step III=== | ===Step III=== | ||
| Line 67: | Line 62: | ||
===Step IV=== | ===Step IV=== | ||
| - | <scene name='90/904321/Iv/1'>Step IV</scene> is the last step of this cycle. Vitamin K Quinone will exit the central binding pocket and the open conformation will form. VKOR is in its fully oxidized state after donating its electrons to Vitamin K Epoxide. This is when the luminal helix will be visible. The cycle then repeats at Step I to keep reducing Vitamin K Epoxide to Vitamin K Quinone. <ref name="Liu"/> | + | <scene name='90/904321/Iv/1'>Step IV</scene> is the last step of this cycle. A bond between Cys43 and Cys51 causes an electron transfer to Cys132. In its reduced form Cys132 will attack Cys135, and the extra electrons are kicked to Vitamin K Epoxide. This opens the epoxide ring on Vitamin K Epoxide so that it may be reformed into Vitamin K Quinone. Vitamin K Quinone is released from Vitamin K Epoxide Reductase. This is step <scene name='90/904321/Iv/1'>IV</scene>, which is a fully oxidized open conformation of VKOR. This process is repeated over and over unless interrupted by inhibitors known as Vitamin K Antagonists or VKAs. Vitamin K Quinone will exit the central binding pocket and the open conformation will form. VKOR is in its fully oxidized state after donating its electrons to Vitamin K Epoxide. This is when the luminal helix will be visible. The cycle then repeats at Step I to keep reducing Vitamin K Epoxide to Vitamin K Quinone. <ref name="Liu"/> |
== Warfarin == | == Warfarin == | ||
[https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common [https://en.wikipedia.org/wiki/Vitamin_K_antagonist Vitamin K antagonist (VKA)]. Warfarin is a competitive inhibitor, taking the place of Vitamin K Epoxide (VKO) in the active site of Vitamin K Epoxide Reductase (VKOR). When warfarin binds in the active site, it causes VKOR to go into the closed conformation. | [https://en.wikipedia.org/wiki/Warfarin Warfarin] is the most common [https://en.wikipedia.org/wiki/Vitamin_K_antagonist Vitamin K antagonist (VKA)]. Warfarin is a competitive inhibitor, taking the place of Vitamin K Epoxide (VKO) in the active site of Vitamin K Epoxide Reductase (VKOR). When warfarin binds in the active site, it causes VKOR to go into the closed conformation. | ||
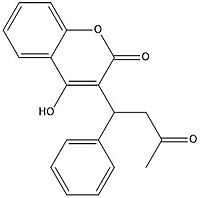
| - | [[Image:warfarin.jpg|200 px|left|thumb|Figure | + | [[Image:warfarin.jpg|200 px|left|thumb|Figure 7. Warfarin structure]] |
=== Binding === | === Binding === | ||
| Line 79: | Line 74: | ||
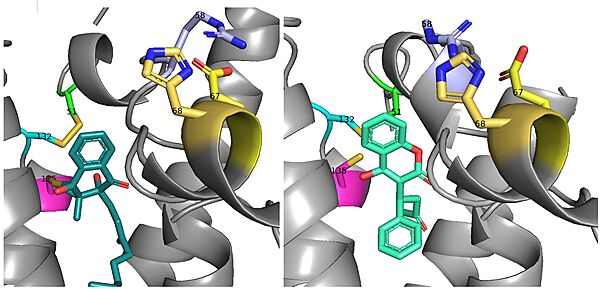
There is a slight difference in the way in which warfarin binds compared to VKO. Warfarin binds are a slightly different angle (Fig.7). This creates a difference in how the cap loop and anchor domain interact, and that noticeable difference is with <scene name='90/904322/Arg58/4'>Arg58</scene>. With VKO, Arg58, located in the cap loop, directly interacts with <scene name='90/904322/Arg58_vko/5'>Glu67</scene> when VKO is bound. When warfarin binds, Arg58 is found inserted between <scene name='90/904322/Arg58_warfarin/2'>Glu67 and His68</scene> of the anchor domain.<ref name="Liu"/> | There is a slight difference in the way in which warfarin binds compared to VKO. Warfarin binds are a slightly different angle (Fig.7). This creates a difference in how the cap loop and anchor domain interact, and that noticeable difference is with <scene name='90/904322/Arg58/4'>Arg58</scene>. With VKO, Arg58, located in the cap loop, directly interacts with <scene name='90/904322/Arg58_vko/5'>Glu67</scene> when VKO is bound. When warfarin binds, Arg58 is found inserted between <scene name='90/904322/Arg58_warfarin/2'>Glu67 and His68</scene> of the anchor domain.<ref name="Liu"/> | ||
| - | [[Image:VKO and Warfarin binding.jpg|600 px|right|thumb|Figure | + | [[Image:VKO and Warfarin binding.jpg|600 px|right|thumb|Figure 8. The slight angle change in which VKO(left) and warfarin(right) bind. The location of the cap domain and how it differs between each is apparent.]] |
Revision as of 15:53, 19 April 2022
Vitamin K Epoxide Reductase
| |||||||||||