We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1722
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
== '''''Human Itch Mas-Related G-Protein Coupled Receptor''''' == | == '''''Human Itch Mas-Related G-Protein Coupled Receptor''''' == | ||
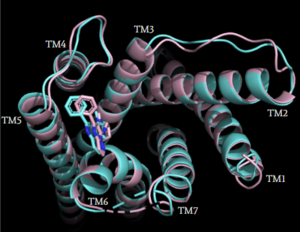
| - | <StructureSection load='' size='350' frame='true' side='right' caption='Structure of MRGPRX2 with transmembrane helices shown in blue, | + | <StructureSection load='' size='350' frame='true' side='right' caption='Structure of MRGPRX2 with transmembrane helices shown in blue. The domains Gαq, Gβ1, and Gγ2 are shown in purple, yellow, and pink, respectively. (PDB entry [https://www.rcsb.org/structure/7S8L 7S8L])' scene='90/904327/Overviewfrontpage/1'> |
== G-Protein Coupled Receptors == | == G-Protein Coupled Receptors == | ||
| + | |||
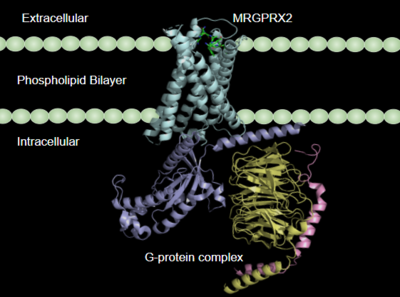
[[Image:Newmembrane.PNG|400px|right|thumb|'''Figure 1''':MRGPRX2 in the cellular membrane. <ref name="Cao"/>]] | [[Image:Newmembrane.PNG|400px|right|thumb|'''Figure 1''':MRGPRX2 in the cellular membrane. <ref name="Cao"/>]] | ||
[https://proteopedia.org/wiki/index.php/G_protein-coupled_receptors G-protein coupled receptors](GCPRs) are a large family of cell surface membrane receptors. Once bound to a wide variety of extracellular ligands, GCPRs undergo a conformational change and relay information to intracellular secondary messengers <ref name="Thal">Thal, David M., et al. "Structural insights into G-protein-coupled receptor allostery." Nature, Nature Publishing Group, 04 July 2018, https://www.nature.com/articles/s41586-018-0259-z</ref>. This G protein activation results in a cellular response dependent on the ligand bound and location of the GPCR in the body. GCPRs can be broken down into five families: the [https://en.wikipedia.org/wiki/Rhodopsin-like_receptors rhodopsin family (class A)], the [https://en.wikipedia.org/wiki/Secretin_receptor_family secretin family (class B)], the [https://en.wikipedia.org/wiki/Adhesion_G_protein-coupled_receptor adhesion family], the [https://en.wikipedia.org/wiki/Class_C_GPCR glutamate family (class C)], and the [https://en.wikipedia.org/wiki/Frizzled frizzled/taste family (class F)] <ref name="Zhang">PMID: 26467290</ref>. All of the families have a similar transmembrane (TM) domain consisting of <scene name='90/904328/7tm_domain_pt_3/7'>seven α-helices</scene> complexed with intracellular G proteins (Figure 1). | [https://proteopedia.org/wiki/index.php/G_protein-coupled_receptors G-protein coupled receptors](GCPRs) are a large family of cell surface membrane receptors. Once bound to a wide variety of extracellular ligands, GCPRs undergo a conformational change and relay information to intracellular secondary messengers <ref name="Thal">Thal, David M., et al. "Structural insights into G-protein-coupled receptor allostery." Nature, Nature Publishing Group, 04 July 2018, https://www.nature.com/articles/s41586-018-0259-z</ref>. This G protein activation results in a cellular response dependent on the ligand bound and location of the GPCR in the body. GCPRs can be broken down into five families: the [https://en.wikipedia.org/wiki/Rhodopsin-like_receptors rhodopsin family (class A)], the [https://en.wikipedia.org/wiki/Secretin_receptor_family secretin family (class B)], the [https://en.wikipedia.org/wiki/Adhesion_G_protein-coupled_receptor adhesion family], the [https://en.wikipedia.org/wiki/Class_C_GPCR glutamate family (class C)], and the [https://en.wikipedia.org/wiki/Frizzled frizzled/taste family (class F)] <ref name="Zhang">PMID: 26467290</ref>. All of the families have a similar transmembrane (TM) domain consisting of <scene name='90/904328/7tm_domain_pt_3/7'>seven α-helices</scene> complexed with intracellular G proteins (Figure 1). | ||
| Line 17: | Line 18: | ||
== Changes in MRGPRX2 from Standard Class A GPCRS == | == Changes in MRGPRX2 from Standard Class A GPCRS == | ||
| + | |||
| + | Class A GPCRs have sequence motifs that have been characterized and are conserved. However, the MRGPRX2 sub-category has the following differences: | ||
==== ''Toggle Switch'' ==== | ==== ''Toggle Switch'' ==== | ||
| - | In β2AR, and other Class A GPCRs, a “toggle switch” of <scene name='90/904327/B2artoggleswitchyes/7'>Trp-286</scene> which limits the proximity of the TM helices as tryptophan sterically occludes tight interaction. This results in a deep binding pocket for ligand binding. In contrast, in MRGPRX2 Trp-286 is replaced by <scene name='90/904327/Toggle_switch_gly_pt_2/2'>Gly-236</scene> <ref name="Cao"/> <ref name="Yang"/>. Glycine is a much smaller amino acid and thus allows the helices to close the base of the binding pocket. This causes MRGPRX2 to have a much shallower binding site and allows more promiscuous ligand binding. This can be seen in a shorter distance from the toggle switch to the ligand in β2AR compared to MRGPRX2. | + | In β2AR, and other Class A GPCRs, a “toggle switch” of <scene name='90/904327/B2artoggleswitchyes/7'>Trp-286</scene> which limits the proximity of the TM helices as tryptophan sterically occludes tight interaction. This results in a deep binding pocket for ligand binding. In contrast, in MRGPRX2 Trp-286 is replaced by <scene name='90/904327/Toggle_switch_gly_pt_2/2'>Gly-236</scene> <ref name="Cao"/> <ref name="Yang"/>. Glycine is a much smaller amino acid and thus allows the helices to close the base of the binding pocket. This causes MRGPRX2 to have a much shallower binding site and allows more promiscuous ligand binding. This can be seen in a shorter distance from the toggle switch to the ligand in β2AR (0.573 nm) compared to MRGPRX2 (1.389 nm). |
==== ''Disulfide bonds'' ==== | ==== ''Disulfide bonds'' ==== | ||
| Line 28: | Line 31: | ||
==== ''PIF Motif'' ==== | ==== ''PIF Motif'' ==== | ||
| - | MRGPRX2 also differs from typical Class A GPCRs based on substitutions in the PIF/connector motif, which acts as a microswitch. PIF plays a role in connecting the binding pocket to conformational rearrangements required for receptor activation<ref name="Schonegge">Schonegge, Anne-Marie, et al. "Evolutionary action and structural basis of the allosteric switch controlling β2AR functional selectivity." Nature, Nature Publishing Group, 18 December 2017, https://www.nature.com/articles/s41467-017-02257-x</ref>. The PIF motif is located towards the base of the TM domains. In a Class A GPCR, like | + | MRGPRX2 also differs from typical Class A GPCRs based on substitutions in the PIF/connector motif, which acts as a microswitch. PIF plays a role in connecting the binding pocket to conformational rearrangements required for receptor activation<ref name="Schonegge">Schonegge, Anne-Marie, et al. "Evolutionary action and structural basis of the allosteric switch controlling β2AR functional selectivity." Nature, Nature Publishing Group, 18 December 2017, https://www.nature.com/articles/s41467-017-02257-x</ref>. The PIF motif is located towards the base of the TM domains. In a Class A GPCR, like <scene name='90/904327/B2arpif_pt_2/1'>β2AR, the PIF motif</scene> consists of Phe-211, Ile-121, and Phe-282 on TM domains 5, 3, and 6, respectively. However, for <scene name='90/904327/Mxpifnolabel_pt_3/1'>MRGPRX2, this PIF motif</scene> is replaced with Leu-194 on TM5, Leu-117 on TM3, and Phe-232 on TM6. |
==== ''DRY Motif'' ==== | ==== ''DRY Motif'' ==== | ||
| Line 59: | Line 62: | ||
=== 2. MRGPRX2 interaction with G-Protein === | === 2. MRGPRX2 interaction with G-Protein === | ||
| + | |||
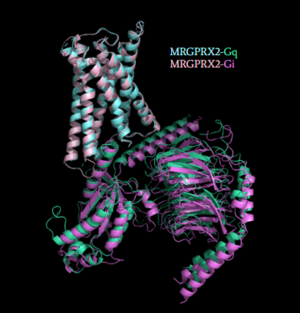
[[Image:Gq_and_Gi_snip.PNG|300px|right|thumb|'''Figure 4''': Caption.<ref name="Cao"/>]] | [[Image:Gq_and_Gi_snip.PNG|300px|right|thumb|'''Figure 4''': Caption.<ref name="Cao"/>]] | ||
| - | Once the ligand is bound, MRGPRX2 undergoes a conformational change that is transmitted through to the [https://en.wikipedia.org/wiki/G_protein#:~:text=G%20proteins%2C%20also%20known%20as,a%20cell%20to%20its%20interior. G-protein]. This conformational change is affected by the aforementioned deviances from Class A GPCRs. Rather than a large conformational change, a subtle one is induced. This will allow MRGPRX2 to interact with a G-protein.<scene name='90/904327/Gproteins/2'>G-proteins</scene>are composed of 3 subunits; α, β, and γ. When activated, the receptor acts as a Guanine nucleotide factor (GEF) which will allow the Gα subunit to have its GDP be replaced by a GTP. This will cause the Gα subunit to dissociate from the dimer Gβγ. The Gα subunit is then able to act as a secondary messenger to begin the signal transduction in the cell. MRGPRX2 interacts with two different types of G-proteins; Gi and Gq. These G-proteins are activated by similar interactions with the receptor however, they are structurally different and changes in the induced conformation are observed (Figure 6). Due to these structural differences, differences in ligand binding to MRGPRX2 bound to Gi in comparison to Gq are observed (Figure 5). | + | Once the ligand is bound, MRGPRX2 undergoes a conformational change that is transmitted through to the [https://en.wikipedia.org/wiki/G_protein#:~:text=G%20proteins%2C%20also%20known%20as,a%20cell%20to%20its%20interior. G-protein]. This conformational change is affected by the aforementioned deviances from Class A GPCRs. Rather than a large conformational change, a subtle one is induced. This will allow MRGPRX2 to interact with a G-protein. <scene name='90/904327/Gproteins/2'>G-proteins</scene> are composed of 3 subunits; α, β, and γ. When activated, the receptor acts as a Guanine nucleotide factor (GEF) which will allow the Gα subunit to have its GDP be replaced by a GTP. This will cause the Gα subunit to dissociate from the dimer Gβγ. The Gα subunit is then able to act as a secondary messenger to begin the signal transduction in the cell. MRGPRX2 interacts with two different types of G-proteins; Gi and Gq. These G-proteins are activated by similar interactions with the receptor however, they are structurally different and changes in the induced conformation are observed (Figure 6). Due to these structural differences, differences in ligand binding to MRGPRX2 bound to Gi in comparison to Gq are observed (Figure 5). |
[[Image:GqGi_with_zincs_snip.PNG|300px|left|thumb|'''Figure 5''': Caption.<ref name="Cao"/>]] | [[Image:GqGi_with_zincs_snip.PNG|300px|left|thumb|'''Figure 5''': Caption.<ref name="Cao"/>]] | ||
| Line 68: | Line 72: | ||
MRGPRX2 also interacts with Gi or G-protein inhibitory, which is structurally homologous to Gs (G-protein stimulatory), and will inhibit adenylyl cyclase and therefore lowers (cAMP). Gi is stimulated when somatostatin binds to receptor in pancreas. | MRGPRX2 also interacts with Gi or G-protein inhibitory, which is structurally homologous to Gs (G-protein stimulatory), and will inhibit adenylyl cyclase and therefore lowers (cAMP). Gi is stimulated when somatostatin binds to receptor in pancreas. | ||
| - | |||
== Clinical Relevance == | == Clinical Relevance == | ||
| Line 77: | Line 80: | ||
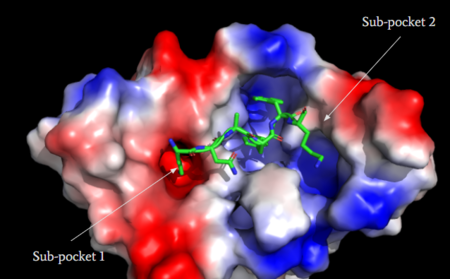
This receptor is shallow and is able to bind to a variety of drugs, creating a need a solution to mediate the effects of MRGPRX2 activation. Currently, there is research for the development of small antagonists that will induce an anti-inflammatory effect by prevent [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE-dependent] mast cell degranulation.<ref name="McNeil">McNeil, B. D., et al. "MRGPRX2 and Adverse Drug Reactions." Frontier Immunology, 06 August 2021, https://www.frontiersin.org/articles/10.3389/fimmu.2021.676354/full</ref> There are two antagonists that have been adapted from being agonists to another Class A GPCR, [https://en.wikipedia.org/wiki/NK1_receptor_antagonist neurokinin-1 receptor (NK-1R)].<ref name="Ogasawara">Ogasawara, H., et al. "Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells." Journal of Leukocyte Biology, 12 July 2019, https://jlb.onlinelibrary.wiley.com/doi/10.1002/JLB.2AB1018-405R</ref> Through the development of this research, these adverse side effects can be relieved and improve patient satisfaction. | This receptor is shallow and is able to bind to a variety of drugs, creating a need a solution to mediate the effects of MRGPRX2 activation. Currently, there is research for the development of small antagonists that will induce an anti-inflammatory effect by prevent [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE-dependent] mast cell degranulation.<ref name="McNeil">McNeil, B. D., et al. "MRGPRX2 and Adverse Drug Reactions." Frontier Immunology, 06 August 2021, https://www.frontiersin.org/articles/10.3389/fimmu.2021.676354/full</ref> There are two antagonists that have been adapted from being agonists to another Class A GPCR, [https://en.wikipedia.org/wiki/NK1_receptor_antagonist neurokinin-1 receptor (NK-1R)].<ref name="Ogasawara">Ogasawara, H., et al. "Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells." Journal of Leukocyte Biology, 12 July 2019, https://jlb.onlinelibrary.wiley.com/doi/10.1002/JLB.2AB1018-405R</ref> Through the development of this research, these adverse side effects can be relieved and improve patient satisfaction. | ||
| - | |||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 22:33, 20 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Human Itch Mas-Related G-Protein Coupled Receptor
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Cao, Can, et al. "Structure, function and pharmacology of human itch GPCRs." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04126-6
- ↑ Thal, David M., et al. "Structural insights into G-protein-coupled receptor allostery." Nature, Nature Publishing Group, 04 July 2018, https://www.nature.com/articles/s41586-018-0259-z
- ↑ 3.0 3.1 Zhang D, Zhao Q, Wu B. Structural Studies of G Protein-Coupled Receptors. Mol Cells. 2015 Oct;38(10):836-42. doi: 10.14348/molcells.2015.0263. Epub 2015, Oct 15. PMID:26467290 doi:http://dx.doi.org/10.14348/molcells.2015.0263
- ↑ 4.0 4.1 4.2 Zhou Q, Yang D, Wu M, Guo Y, Guo W, Zhong L, Cai X, Dai A, Jang W, Shakhnovich EI, Liu ZJ, Stevens RC, Lambert NA, Babu MM, Wang MW, Zhao S. Common activation mechanism of class A GPCRs. Elife. 2019 Dec 19;8. pii: 50279. doi: 10.7554/eLife.50279. PMID:31855179 doi:http://dx.doi.org/10.7554/eLife.50279
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y
- ↑ 6.0 6.1 Schonegge, Anne-Marie, et al. "Evolutionary action and structural basis of the allosteric switch controlling β2AR functional selectivity." Nature, Nature Publishing Group, 18 December 2017, https://www.nature.com/articles/s41467-017-02257-x
- ↑ Sandoval, A., et al. "The Molecular Switching Mechanism at the Conserved D(E)RY Motif in Class-A GPCRs." Biophysical journal, 111(1), 79-89. https://doi.org/10.1016/j.bpj.2016.06.004
- ↑ Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014 May;39(5):233-44. doi: 10.1016/j.tibs.2014.03.002. Epub , 2014 Apr 21. PMID:24767681 doi:http://dx.doi.org/10.1016/j.tibs.2014.03.002
- ↑ Babina, M., et al. "MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway." Journal of Investigative Dermatology, 141(6), 1286-1296. https://doi.org/10.1016/j.jid.2020.09.017
- ↑ McNeil, B. D., et al. "MRGPRX2 and Adverse Drug Reactions." Frontier Immunology, 06 August 2021, https://www.frontiersin.org/articles/10.3389/fimmu.2021.676354/full
- ↑ Ogasawara, H., et al. "Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells." Journal of Leukocyte Biology, 12 July 2019, https://jlb.onlinelibrary.wiley.com/doi/10.1002/JLB.2AB1018-405R