Neurofibromin
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
===Domains=== | ===Domains=== | ||
<scene name='90/904326/Overview_of_domains/1'>Neurofibromin</scene> consists of multiple domains. (Figure 1). A few notable ones are the N-HEAT/ARM, GRD, Sec14-PH, and C-HEAT/ARM. The two most characterized domains of neurofibromin are the Sec14-PH and GRD domains. Each of the protomers of neurofibromin contains these domains. | <scene name='90/904326/Overview_of_domains/1'>Neurofibromin</scene> consists of multiple domains. (Figure 1). A few notable ones are the N-HEAT/ARM, GRD, Sec14-PH, and C-HEAT/ARM. The two most characterized domains of neurofibromin are the Sec14-PH and GRD domains. Each of the protomers of neurofibromin contains these domains. | ||
| - | [[Image:domainsneurofibromin.png|500 px|thumb|Figure 1. Domains of Neurofibromin.]] | + | [[Image:domainsneurofibromin.png|500 px|thumb|Figure 1. Representation of the Domains of Neurofibromin. Shown are the most characterized domains, Gap-related and Sec14-PH, connected to the N and C-HEAT/ARMs. Each of the domains of Neurofibromin can be found in both of the monomers.]] |
====N-HEAT/ARM and C-HEAT/ARM==== | ====N-HEAT/ARM and C-HEAT/ARM==== | ||
[https://en.wikipedia.org/wiki/HEAT_repeat Heat domains] are domains found in cytoplasmic proteins that consist of four different proteins: [https://proteopedia.org/wiki/index.php/Huntingtin Huntingtin], [https://proteopedia.org/wiki/index.php/Elongation_factor elongation factor 3], [https://proteopedia.org/wiki/index.php/Protein_phosphatase protein phosphatase 2A], and TOR1. <ref name= ''Yoshimura''>DOI: 10.1242/jcs.185710</ref>. The [https://en.wikipedia.org/wiki/HEAT_repeat HEAT] / [https://en.wikipedia.org/wiki/Armadillo_repeat ARM] cores are made up of many alpha helices. The N-HEAT/ARM and C-HEAT/ARM are rigid, which makes them critical in the rearrangement of the Gap-related and Sec14-PH domains. In the <scene name='90/904326/Heat/1'>closed conformation</scene>, the HEAT/ARM domains cover the GRD, preventing the binding of Ras through steric hinderance. <ref name= "Lupton">DOI 10.1038/s41594-021-00687-2</ref> | [https://en.wikipedia.org/wiki/HEAT_repeat Heat domains] are domains found in cytoplasmic proteins that consist of four different proteins: [https://proteopedia.org/wiki/index.php/Huntingtin Huntingtin], [https://proteopedia.org/wiki/index.php/Elongation_factor elongation factor 3], [https://proteopedia.org/wiki/index.php/Protein_phosphatase protein phosphatase 2A], and TOR1. <ref name= ''Yoshimura''>DOI: 10.1242/jcs.185710</ref>. The [https://en.wikipedia.org/wiki/HEAT_repeat HEAT] / [https://en.wikipedia.org/wiki/Armadillo_repeat ARM] cores are made up of many alpha helices. The N-HEAT/ARM and C-HEAT/ARM are rigid, which makes them critical in the rearrangement of the Gap-related and Sec14-PH domains. In the <scene name='90/904326/Heat/1'>closed conformation</scene>, the HEAT/ARM domains cover the GRD, preventing the binding of Ras through steric hinderance. <ref name= "Lupton">DOI 10.1038/s41594-021-00687-2</ref> | ||
| Line 18: | Line 18: | ||
=====Closed Conformation===== | =====Closed Conformation===== | ||
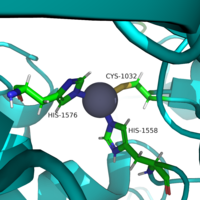
The <scene name='90/904326/Overview_of_domains/1'>closed state</scene> of neurofibromin has both protomers in a closed conformation, which inhibits the binding of Ras to the GRD of neurofibromin due to the HEAT/ARM core blocking the GRD. A metal binding site between the N-HEAT/ARM domain and the GRD-Sec14-PH linker stabilize the closed conformation. This site is coordinated by three residues, <scene name='90/904325/Triad/1'>C1032, H1558, and H1576,</scene>. (Figure 2). This binding site is preferential for zinc- zinc has been found to stabilize the closed conformation of neurofibromin. In the absence of zinc, neurofibromin is in the open conformation. <ref name="Naschberger"/> | The <scene name='90/904326/Overview_of_domains/1'>closed state</scene> of neurofibromin has both protomers in a closed conformation, which inhibits the binding of Ras to the GRD of neurofibromin due to the HEAT/ARM core blocking the GRD. A metal binding site between the N-HEAT/ARM domain and the GRD-Sec14-PH linker stabilize the closed conformation. This site is coordinated by three residues, <scene name='90/904325/Triad/1'>C1032, H1558, and H1576,</scene>. (Figure 2). This binding site is preferential for zinc- zinc has been found to stabilize the closed conformation of neurofibromin. In the absence of zinc, neurofibromin is in the open conformation. <ref name="Naschberger"/> | ||
| - | [[Image:greenTriad.png|200 px|thumb|Figure 2. Triad of Residues that keep Neurofibromin in the Closed Conformation.]] | + | [[Image:greenTriad.png|200 px|thumb|Figure 2. Triad of Residues that keep Neurofibromin in the Closed Conformation. The residues shown are C1032, H1558, and H1576. In the center of those residues is a zinc ion, shown in grey.]] |
=====Open Conformation===== | =====Open Conformation===== | ||
The <scene name='90/904326/Open/1'>open state</scene> of neurofibromin has one protomer in a open conformation and the other in a closed conformation. The protomer in the open conformation allows for the binding of Ras because of reorientation of the GRD and Sec14-PH domains. In the open conformation, the metal binding site found in the closed conformation is lost due to separation of the N-HEAT/ARM and the cysteine residue from the histidine residues founds in the GRD-Sec14-PH linker. The loss of the metal binding site in one of the monomers allows for Ras to bind to the Gap-related domain due to the loss of steric hinderance. | The <scene name='90/904326/Open/1'>open state</scene> of neurofibromin has one protomer in a open conformation and the other in a closed conformation. The protomer in the open conformation allows for the binding of Ras because of reorientation of the GRD and Sec14-PH domains. In the open conformation, the metal binding site found in the closed conformation is lost due to separation of the N-HEAT/ARM and the cysteine residue from the histidine residues founds in the GRD-Sec14-PH linker. The loss of the metal binding site in one of the monomers allows for Ras to bind to the Gap-related domain due to the loss of steric hinderance. | ||
Revision as of 12:56, 21 April 2022
| |||||||||||
References
- ↑ 1.0 1.1 Bergoug M, Doudeau M, Godin F, Mosrin C, Vallee B, Benedetti H. Neurofibromin Structure, Functions and Regulation. Cells. 2020 Oct 27;9(11). pii: cells9112365. doi: 10.3390/cells9112365. PMID:33121128 doi:http://dx.doi.org/10.3390/cells9112365
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Naschberger A, Baradaran R, Rupp B, Carroni M. The structure of neurofibromin isoform 2 reveals different functional states. Nature. 2021 Nov;599(7884):315-319. doi: 10.1038/s41586-021-04024-x. Epub 2021, Oct 27. PMID:34707296 doi:http://dx.doi.org/10.1038/s41586-021-04024-x
- ↑ Trovo-Marqui AB, Tajara EH. Neurofibromin: a general outlook. Clin Genet. 2006 Jul;70(1):1-13. doi: 10.1111/j.1399-0004.2006.00639.x. PMID:16813595 doi:http://dx.doi.org/10.1111/j.1399-0004.2006.00639.x
- ↑ Hall BE, Bar-Sagi D, Nassar N. The structural basis for the transition from Ras-GTP to Ras-GDP. Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12138-42. Epub 2002 Sep 4. PMID:12213964 doi:http://dx.doi.org/10.1073/pnas.192453199
- ↑ Cimino PJ, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol. 2018;148:799-811. doi: 10.1016/B978-0-444-64076-5.00051-X. PMID:29478615 doi:http://dx.doi.org/10.1016/B978-0-444-64076-5.00051-X
- ↑ Yoshimura SH, Hirano T. HEAT repeats - versatile arrays of amphiphilic helices working in crowded environments? J Cell Sci. 2016 Nov 1;129(21):3963-3970. doi: 10.1242/jcs.185710. Epub 2016 Oct , 6. PMID:27802131 doi:http://dx.doi.org/10.1242/jcs.185710
- ↑ 7.0 7.1 7.2 Lupton CJ, Bayly-Jones C, D'Andrea L, Huang C, Schittenhelm RB, Venugopal H, Whisstock JC, Halls ML, Ellisdon AM. The cryo-EM structure of the human neurofibromin dimer reveals the molecular basis for neurofibromatosis type 1. Nat Struct Mol Biol. 2021 Dec;28(12):982-988. doi: 10.1038/s41594-021-00687-2., Epub 2021 Dec 9. PMID:34887559 doi:http://dx.doi.org/10.1038/s41594-021-00687-2
- ↑ Scheffzek K, Welti S. Pleckstrin homology (PH) like domains - versatile modules in protein-protein interaction platforms. FEBS Lett. 2012 Aug 14;586(17):2662-73. doi: 10.1016/j.febslet.2012.06.006. Epub , 2012 Jun 19. PMID:22728242 doi:http://dx.doi.org/10.1016/j.febslet.2012.06.006
- ↑ Dunzendorfer-Matt T, Mercado EL, Maly K, McCormick F, Scheffzek K. The neurofibromin recruitment factor Spred1 binds to the GAP related domain without affecting Ras inactivation. Proc Natl Acad Sci U S A. 2016 Jul 5;113(27):7497-502. doi:, 10.1073/pnas.1607298113. Epub 2016 Jun 16. PMID:27313208 doi:http://dx.doi.org/10.1073/pnas.1607298113
- ↑ Frech M, Darden TA, Pedersen LG, Foley CK, Charifson PS, Anderson MW, Wittinghofer A. Role of glutamine-61 in the hydrolysis of GTP by p21H-ras: an experimental and theoretical study. Biochemistry. 1994 Mar 22;33(11):3237-44. doi: 10.1021/bi00177a014. PMID:8136358 doi:http://dx.doi.org/10.1021/bi00177a014
- ↑ Bunda S, Burrell K, Heir P, Zeng L, Alamsahebpour A, Kano Y, Raught B, Zhang ZY, Zadeh G, Ohh M. Inhibition of SHP2-mediated dephosphorylation of Ras suppresses oncogenesis. Nat Commun. 2015 Nov 30;6:8859. doi: 10.1038/ncomms9859. PMID:26617336 doi:http://dx.doi.org/10.1038/ncomms9859
- ↑ Abramowicz A, Gos M. Neurofibromin in neurofibromatosis type 1 - mutations in NF1gene as a cause of disease. Dev Period Med. 2014 Jul-Sep;18(3):297-306. PMID:25182393
- ↑ Cimino PJ, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol. 2018;148:799-811. doi: 10.1016/B978-0-444-64076-5.00051-X. PMID:29478615 doi:http://dx.doi.org/10.1016/B978-0-444-64076-5.00051-X
- ↑ Ly KI, Blakeley JO. The Diagnosis and Management of Neurofibromatosis Type 1. Med Clin North Am. 2019 Nov;103(6):1035-1054. doi: 10.1016/j.mcna.2019.07.004. PMID:31582003 doi:http://dx.doi.org/10.1016/j.mcna.2019.07.004
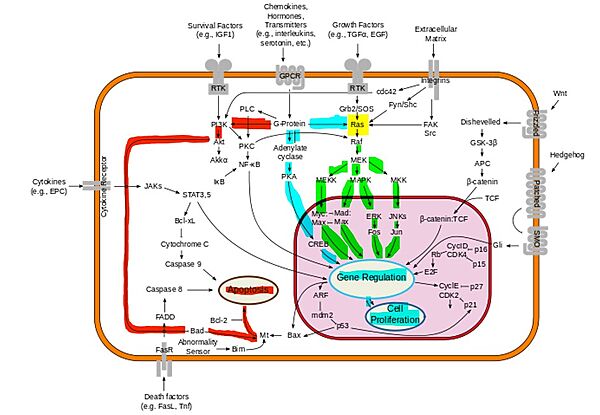
- ↑ McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007 Aug;1773(8):1263-84. doi:, 10.1016/j.bbamcr.2006.10.001. Epub 2006 Oct 7. PMID:17126425 doi:http://dx.doi.org/10.1016/j.bbamcr.2006.10.001
Proteopedia Page Contributors and Editors (what is this?)
Jordyn K. Lenard, Ryan D. Adkins, Michal Harel, OCA, Jaime Prilusky