We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1722
From Proteopedia
(Difference between revisions)
| Line 53: | Line 53: | ||
==== Agonists ==== | ==== Agonists ==== | ||
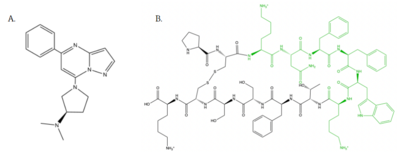
| - | [[Image:Agoniststogether.PNG |400px|right|thumb|'''Figure 4''': Structure of MRGPRX2 Agonists. (A) Structure of R-Zinc 3573. A cationic ligand selected for binding to MRGPRX2 <ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref>.(B) Structure of Cortistatin-14 with resolved amino acids highlighted in green. These ligands were used as a probes for MRGPRX2 function and stabilization for structure determination <ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref>.]] | + | [[Image:Agoniststogether.PNG |400px|right|thumb|'''Figure 4''': Structure of MRGPRX2 Agonists. (A) Structure of R-Zinc 3573. A cationic ligand selected for binding to MRGPRX2 <ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref>. (B) Structure of Cortistatin-14 with resolved amino acids highlighted in green. These ligands were used as a probes for MRGPRX2 function and stabilization for structure determination <ref name="Yang">Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y</ref>.]] |
===== Small Molecule ===== | ===== Small Molecule ===== | ||
| Line 76: | Line 76: | ||
== Clinical Relevance == | == Clinical Relevance == | ||
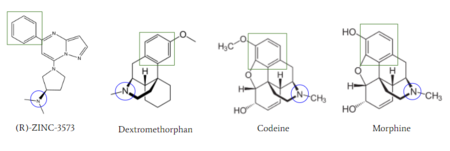
| - | [[Image:Zinc_and_drugs_snip.PNG|450px|right|thumb|'''Figure 6''': Structures of (R)-Zinc-3573, Dextromethorphan, Morphine, and Codeine. The blue circles indicate conserved basic N-dimethyl group and the green squares show the conserved benzene rings | + | [[Image:Zinc_and_drugs_snip.PNG|450px|right|thumb|'''Figure 6''': Structures of (R)-Zinc-3573, Dextromethorphan, Morphine, and Codeine. The blue circles indicate conserved basic N-dimethyl group and the green squares show the conserved benzene rings <ref name="Cao"/>.]] |
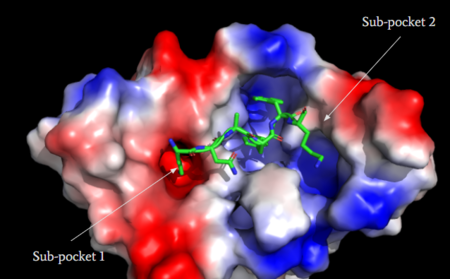
MRGPRX2 activation is associated with chronic itching or anaphylaxis reactions, a common side effect of many prescribed medications <ref name="Cao"/>. Among these drugs are opioids [https://en.wikipedia.org/wiki/Morphine morphine] and [https://en.wikipedia.org/wiki/Codeine codeine] and [https://en.wikipedia.org/wiki/Dextromethorphan dextromethorphan]. By analyzing the structures of these drugs, it can be seen that they contain chemical features similar to <scene name='90/904328/Zizwithaa/5'>agonist R-Zinc-3573</scene> (see Figure 6). They contain a conserved benzene ring that stabilizes them in binding pocket 1. Similarly, they contain an N-dimethyl group that would allow them to form key ionic bonds with residues Asp-184 and Glu-164. Lastly, these drugs have a similar shape and size to that of the agonist R-Zinc-3573 <ref name="Babina"> Babina, M., et al. "MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway." Journal of Investigative Dermatology, 141(6), 1286-1296. https://doi.org/10.1016/j.jid.2020.09.017</ref> (see Figure 6). These structural and chemical similarities indicate the possibility of a similar binding mechanism to previously discussed agonist, (R)-Zinc-3573. | MRGPRX2 activation is associated with chronic itching or anaphylaxis reactions, a common side effect of many prescribed medications <ref name="Cao"/>. Among these drugs are opioids [https://en.wikipedia.org/wiki/Morphine morphine] and [https://en.wikipedia.org/wiki/Codeine codeine] and [https://en.wikipedia.org/wiki/Dextromethorphan dextromethorphan]. By analyzing the structures of these drugs, it can be seen that they contain chemical features similar to <scene name='90/904328/Zizwithaa/5'>agonist R-Zinc-3573</scene> (see Figure 6). They contain a conserved benzene ring that stabilizes them in binding pocket 1. Similarly, they contain an N-dimethyl group that would allow them to form key ionic bonds with residues Asp-184 and Glu-164. Lastly, these drugs have a similar shape and size to that of the agonist R-Zinc-3573 <ref name="Babina"> Babina, M., et al. "MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway." Journal of Investigative Dermatology, 141(6), 1286-1296. https://doi.org/10.1016/j.jid.2020.09.017</ref> (see Figure 6). These structural and chemical similarities indicate the possibility of a similar binding mechanism to previously discussed agonist, (R)-Zinc-3573. | ||
Revision as of 15:28, 21 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
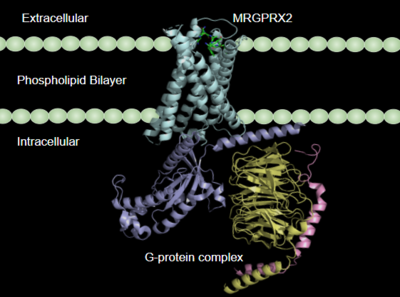
Human Itch Mas-Related G-Protein Coupled Receptor
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Cao, Can, et al. "Structure, function and pharmacology of human itch GPCRs." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04126-6
- ↑ Thal, David M., et al. "Structural insights into G-protein-coupled receptor allostery." Nature, Nature Publishing Group, 04 July 2018, https://www.nature.com/articles/s41586-018-0259-z
- ↑ 3.0 3.1 Zhang D, Zhao Q, Wu B. Structural Studies of G Protein-Coupled Receptors. Mol Cells. 2015 Oct;38(10):836-42. doi: 10.14348/molcells.2015.0263. Epub 2015, Oct 15. PMID:26467290 doi:http://dx.doi.org/10.14348/molcells.2015.0263
- ↑ 4.0 4.1 Ramesh, Soliman, et al. (2015) "G-Protein Coupled Receptors (GPCRs): A Comprehensive Computational Perspective." Combinational Chemistry and High Throughout Screening, 18(4), 346-364, https://pubmed.ncbi.nlm.nih.gov/25747435/
- ↑ 5.0 5.1 5.2 5.3 Zhou Q, Yang D, Wu M, Guo Y, Guo W, Zhong L, Cai X, Dai A, Jang W, Shakhnovich EI, Liu ZJ, Stevens RC, Lambert NA, Babu MM, Wang MW, Zhao S. Common activation mechanism of class A GPCRs. Elife. 2019 Dec 19;8. pii: 50279. doi: 10.7554/eLife.50279. PMID:31855179 doi:http://dx.doi.org/10.7554/eLife.50279
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y
- ↑ 7.0 7.1 Schonegge, Anne-Marie, et al. "Evolutionary action and structural basis of the allosteric switch controlling β2AR functional selectivity." Nature, Nature Publishing Group, 18 December 2017, https://www.nature.com/articles/s41467-017-02257-x
- ↑ Sandoval, A., et al. "The Molecular Switching Mechanism at the Conserved D(E)RY Motif in Class-A GPCRs." Biophysical journal, 111(1), 79-89. https://doi.org/10.1016/j.bpj.2016.06.004
- ↑ Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014 May;39(5):233-44. doi: 10.1016/j.tibs.2014.03.002. Epub , 2014 Apr 21. PMID:24767681 doi:http://dx.doi.org/10.1016/j.tibs.2014.03.002
- ↑ Babina, M., et al. "MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway." Journal of Investigative Dermatology, 141(6), 1286-1296. https://doi.org/10.1016/j.jid.2020.09.017
- ↑ McNeil, B. D., et al. "MRGPRX2 and Adverse Drug Reactions." Frontier Immunology, 06 August 2021, https://www.frontiersin.org/articles/10.3389/fimmu.2021.676354/full
- ↑ Ogasawara, H., et al. "Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells." Journal of Leukocyte Biology, 12 July 2019, https://jlb.onlinelibrary.wiley.com/doi/10.1002/JLB.2AB1018-405R