User:Camryn Jiles/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
[[Image:IMG_2893.jpg | thumb]] | [[Image:IMG_2893.jpg | thumb]] | ||
== Relevance == | == Relevance == | ||
| + | Expression of orexin receptors isn’t just limited to the brain. They can be found in the adrenal glands, pancreas, vertebrate testis, placenta, kidneys and more. Stress is a major stimulator for increased orexin production, as it induces the release of cortisol from the adrenals. The relationship between orexin and stress is inversely proportional, and regulation of acute stress is better understood then long term stress; “orexin promote the acute behavioral and neuroendocrine response to stress, and acute stress activates orexin neurons” (Grafe). Glucose is another stimulator of orexin release and comes from the beta cells of the pancreas. Whenever the body is hungry, the plasma concentration of orexin increases which subsequently increases appetite. Though the entire process of how this works is still misunderstood, it is suggested that ghrelin (hunger hormone) activates orexin neurons independent of NPY. There is also a relationship between obesity and orexin worth briefly noting. A recent study showed that orexin increases adipocyte glucose uptake and stimulates adiponectin release in rats. Additionally, lipoprotein lipase in adipocytes increases when exposed to orexin. In conclusion, this study showed that orexin increases the enzyme that degrades fat hence, removal of orexin causes obesity due to decreased energy and browning of fat (Adeghate 6). | ||
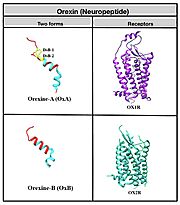
| + | GPCR’s are the largest, diverse family of human transmembrane proteins and play an important role in cell signaling. This factor makes them ideal for drug targets to achieve numerous therapeutic effects; with this in mind it is important to get a broad understanding of the structure and binding dynamics of GPCRs. OX1R and OX2R are class A GPCRs which is the largest of the four classes, and most diverse in humans. Its structure consists of a “seven-transmembrane helices domain, three extracellular loops and three intracellular loops with ligand binding pockets” (Vu 1). Peptide hormones like neuropeptides, though very diverse, undergo similar processing to get to their active form; many undergo different post-translational modifications to achieve this by inhibiting exopeptidase, bromination, lipidation, disulfide bridge formation or differential proteolysis. Neuropeptides specifically undergo C-terminal amidation which acts to reduce the charge of the peptide which improves potency, metabolic stability and increases ability to resist enzymatic degradation. | ||
== Inhibition == | == Inhibition == | ||
| - | + | Due to orexins' strong regulation on sleep, decreased production can lead to narcolepsy. Narcolepsy is a chronic sleep disorder characterized by overwhelming drowsiness and sudden attacks of sleep. In addition, dysregulation can lead to extreme happiness or extreme sadness due to orexin's effect on mood. Abnormal expression of orexin receptor type 1 can cause cancer and the development of metabolic diseases, like diabetes, can also occur. However, GPCR peptide drugs can aid in reducing these symptoms by modulating the orexin signaling pathway– ultimately inhibiting it. Antagonistic drugs called orexin-receptor antagonists (SORAs) have been made to target orexin receptors, consisting of the antagonist compound suvorexant. Upon binding, suvorexant adopts a π-stacked conformation and binds deep to the active site of the receptor and prevents transmembrane helix activation. A molecular binding simulation between OxB and OX1R showed that “mutation in the alanine residue of K120, P123, Y124, N318, F340, T341, H344 and W345 located in the TM2, TM3, TM6 and TM7 reduces binding affinity and inactivates the intracellular release of calcium ions. | |
== Conclusion == | == Conclusion == | ||
| - | + | In conclusion, orexin and its receptors play a major role in maintaining overall homeostasis within the body. Its involvement in various neural activity, including other organ systems makes it an important biochemical protein to obtain feeding behaviors, energy balance, the sleep-wake cycle and more. One future area of study that may be interesting to explore would be further understanding of the relationship between orexin and long term stress, as it is misunderstood at the present time. | |
Revision as of 17:20, 25 April 2022
Neuropeptide: Orexin
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644