User:Camryn Jiles/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
== Inhibition == | == Inhibition == | ||
Due to orexins' strong regulation on sleep, decreased production can lead to narcolepsy. Narcolepsy is a chronic sleep disorder characterized by overwhelming drowsiness and sudden attacks of sleep. In addition, dysregulation can lead to extreme happiness or extreme sadness due to orexin's effect on mood. Abnormal expression of orexin receptor type 1 can cause cancer and the development of metabolic diseases, like diabetes, can also occur. However, GPCR peptide drugs can aid in reducing these symptoms by modulating the orexin signaling pathway– ultimately inhibiting it. Antagonistic drugs called orexin-receptor antagonists (SORAs) have been made to target orexin receptors, consisting of the antagonist compound suvorexant. Upon binding, suvorexant adopts a π-stacked conformation and binds deep to the active site of the receptor and prevents transmembrane helix activation. A molecular binding simulation between OxB and OX1R showed that “mutation in the alanine residue of K120, P123, Y124, N318, F340, T341, H344 and W345 located in the TM2, TM3, TM6 and TM7 reduces binding affinity and inactivates the intracellular release of calcium ions. | Due to orexins' strong regulation on sleep, decreased production can lead to narcolepsy. Narcolepsy is a chronic sleep disorder characterized by overwhelming drowsiness and sudden attacks of sleep. In addition, dysregulation can lead to extreme happiness or extreme sadness due to orexin's effect on mood. Abnormal expression of orexin receptor type 1 can cause cancer and the development of metabolic diseases, like diabetes, can also occur. However, GPCR peptide drugs can aid in reducing these symptoms by modulating the orexin signaling pathway– ultimately inhibiting it. Antagonistic drugs called orexin-receptor antagonists (SORAs) have been made to target orexin receptors, consisting of the antagonist compound suvorexant. Upon binding, suvorexant adopts a π-stacked conformation and binds deep to the active site of the receptor and prevents transmembrane helix activation. A molecular binding simulation between OxB and OX1R showed that “mutation in the alanine residue of K120, P123, Y124, N318, F340, T341, H344 and W345 located in the TM2, TM3, TM6 and TM7 reduces binding affinity and inactivates the intracellular release of calcium ions. | ||
| + | |||
| + | <scene name='91/910030/Suvorexant_antagonist/1'>OX2 receptor bound to suvorexant</scene> | ||
== Conclusion == | == Conclusion == | ||
In conclusion, orexin and its receptors play a major role in maintaining overall homeostasis within the body. Its involvement in various neural activity, including other organ systems makes it an important biochemical protein to obtain feeding behaviors, energy balance, the sleep-wake cycle and more. One future area of study that may be interesting to explore would be further understanding of the relationship between orexin and long term stress, as it is misunderstood at the present time. | In conclusion, orexin and its receptors play a major role in maintaining overall homeostasis within the body. Its involvement in various neural activity, including other organ systems makes it an important biochemical protein to obtain feeding behaviors, energy balance, the sleep-wake cycle and more. One future area of study that may be interesting to explore would be further understanding of the relationship between orexin and long term stress, as it is misunderstood at the present time. | ||
Revision as of 17:50, 25 April 2022
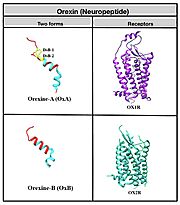
Neuropeptide: Orexin
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644