Ricin: Structure and function
From Proteopedia
| Line 10: | Line 10: | ||
== Structure == | == Structure == | ||
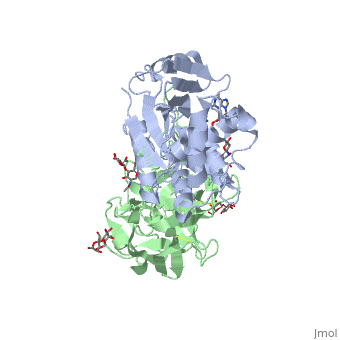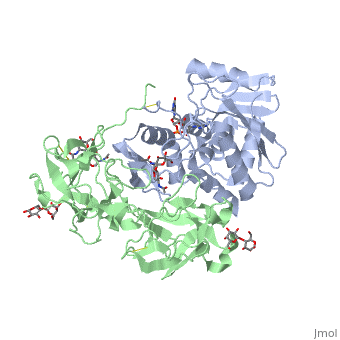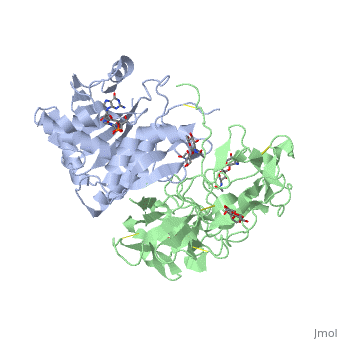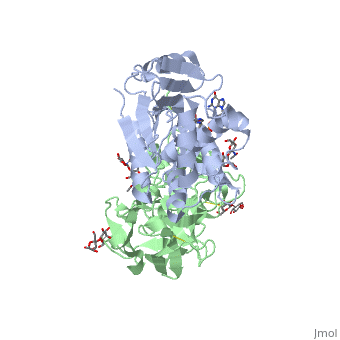
| + | The structure of Ricin consists of two polypeptide chains known as the A and B chain that carry out different functions within the protein due to their amino acid sequences. This heterodimer’s chains are joined by disulfide bonds and have fairly similar molecular weights. In order for ricin to carry out its function, the two chains need to be linked, although they do separate further into the mechanism of action. | ||
| + | The A chain (Ricin A) is a globular protein that consists of 8 beta sheets and 8 alpha helices. It is composed of 267 amino acids and is separated into three structural domains. The first domain is made up of parallel and antiparallel beta sheets, the second contains alpha helices, and the third consists of both. These three domains carry out the function of ribosome inactivation within the Golgi apparatus in a cell, as the active site for depurination lies on the second alpha helical domain. The enzymatic activity of this protein is mainly seen on this chain in the ricin loop, where an adenine of a target cell becomes trapped by two tyrosines. This will result in the prevention of mRNA translation. | ||
| + | The B chain (Ricin B) is a lectin that is composed of 262 amino acids and carries out the action of binding through a galactose binding terminal. This chain does not have any alpha helical or beta sheet structure but does fold into two domains. | ||
| + | These chains are not exclusive to castor plants and they can be found in other non toxic vegetation as well; it is only when these two chains are bound together where they are able to have devastating impacts on an organism. | ||
== Mechanism of Action == | == Mechanism of Action == | ||
| - | + | When the A chain and B chain are joined, they are able to work together to carry out enzymatic activity within an organism. Because they have two completely different properties, their individual functions work together. The main goal of ricin is to inhibit protein synthesis, a process that heavily relies on organelles called ribosomes. When these ribosomes are damaged or inactivated, this halts protein synthesis within a cell. | |
| + | At the start of the process, the B chain’s purpose is to gain entry into a target cell so that the A chain can carry out ribosomal deactivation. As Ricin B attaches to the galactose residues on a cell membrane, the protein is able to invade the cell through the cleavage of the cell membrane, endocytosis. Now that the B chain has done its part in the protein’s function, Chain A has entry into the cytosol of the cell. | ||
| + | After Ricin enters the cell, the disulfide bond between the A and B chains is cleaved and the A chain is released into the cell, working its way to the Golgi network. Ricin A functions to deactivate ribosomal activity through elongation. The enzymatic activity of this protein can inactivate thousands of ribosomes per minute, making it a very fast acting protein. As stated in the structure of the protein, there is a ricin loop on chain A where two tyrosine residues play an important role in the deactivation of the ribosome. Tyr80 and Tyr123 sandwich an adenine to inhibit further protein synthesis. After protein production in a cell is stopped, it is unclear whether the two chains link back together and move on to another cell or whether other cells are affected by new ricin proteins. | ||
== Ricin Poisoning: Symptoms == | == Ricin Poisoning: Symptoms == | ||
| + | Now that we understand the structure and mechanism of action behind Ricin, how can we make sure we take the correct steps in identifying if you may have been exposed to this protein? The main mechanism of this protein is its ability to halt protein synthesis. Because it doesn’t damage the cell directly and instead stops it’s functioning, the symptoms of ricin poisoning can take up to a couple days to be noticed, depending on exposure and dose. | ||
| + | When ricin is ingested, it first manifests symptoms within the gastrointestinal tract, leading to pain and inflammation. The longer an individual is left untreated, the more severe symptoms they may experience. Gastrointestinal symptoms may progress to vomiting and blood in the stool. The loss of blood and fluid due to the symptoms can lead to more detrimental impacts as the body may start to experience the effects of dehydration and hypovolemia. When the body has low blood and water levels, it can result in organ failure and if severe enough, result in death. | ||
| + | The inhalation of ricin can be much more lethal than ingestion as the respiratory system has no action to digest the protein like the digestive system does. Early symptoms can be displayed through throat and lung irritation such as a cough. As the poisoning progresses, it creates an allergic reaction that can cause skin irritation, chest tightness, sore throat, and asthma-like breathing. Oftentimes inhalation symptoms can become severe enough to cause chronic pulmonary disease or vascular leak syndromes. | ||
| + | Due to the early symptoms of ricin poisoning being pretty general and common symptoms associated with common and acute illnesses, doctors can run several diagnosis tests if ricin exposure is suspected. The most common test that can detect the ricin protein in an organism is enzyme-linked immunosorbent assays, also known as the ELISA test. Other methods include testing urine or checking Ricin-antibody counts. Although there are several ways to detect if a person may be experiencing Ricin Poisoning, there is no single method that is used in standard practice as of now. | ||
== Ricin Poisoning: Treatment == | == Ricin Poisoning: Treatment == | ||
Revision as of 18:04, 26 April 2022
| |||||||||||
References
[1]Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin Poisoning: A Comprehensive Review. JAMA. 2005;294(18):2342–2351. doi:10.1001/jama.294.18.2342
[2]Etimad, L., Moshiri, M., & Hamid, F. (2019, June 6). Ricin: An ancient story for a timeless plant toxin. RBMB. Retrieved April 23, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6628454/#sec2-toxins-11-00324title
[3]Gal, Y., Mazor, O., Falach, R., Sapoznikov, A., Kronman, C., & Sabo, T. (2017). Treatments for Pulmonary Ricin Intoxication: Current Aspects and Future Prospects. Toxins, 9(10). https://doi-org.proxy.library.maryville.edu/10.3390/toxins910031
[4]Tumer, N. E. (2019). Introduction to the Toxins Special Issue “Ricin Toxins.” Toxins, 12(1). https://doi-org.proxy.library.maryville.edu/10.3390/toxins12010013/