T. brucei Phosphodiesterase B1
From Proteopedia
| Line 27: | Line 27: | ||
|} | |} | ||
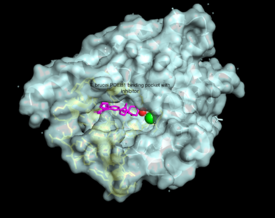
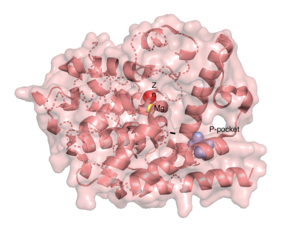
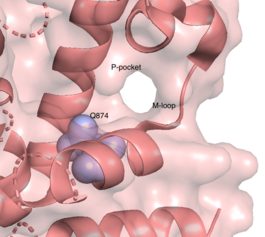
| - | The resolved crystal structure for the catalytic domain of TbPDEB1 (PDB identifier: 4I15) confirmed the existence of a ''T. brucei'' specific '''P-pocket''', a new and exciting direction for improving drug selectivity for ''T. brucei'' | + | The resolved crystal structure for the catalytic domain of TbPDEB1 (PDB identifier: 4I15) confirmed the existence of a ''T. brucei'' specific '''P-pocket''', a new and exciting direction for improving drug selectivity for ''T. brucei'' phosphodiesterase. Following the identification of the parasite specific P-pocket just adjacent to the enzyme binding pocket, new TbPDEB1 inhibitor analogues were created to extend into the parasite enzyme p-pocket. This resulted in improved selectivity for ''T. brucei'' PDEB1 over human PDE4, another cAMP specific phosphodiesterase. |
{| | {| | ||
| [[Image:CatalyticDomain2.png|thumb|right|300x300px|Pymool structure of TbPDE1 catalytic domain]] | | [[Image:CatalyticDomain2.png|thumb|right|300x300px|Pymool structure of TbPDE1 catalytic domain]] | ||
Revision as of 15:13, 30 April 2022
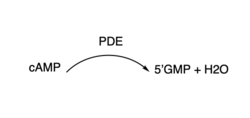
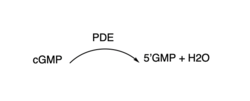
3′,5′-cyclic nucleotide phosphodiesterases (PDEs) are enzymes that hydrolyse 3',5'-phosphodiester bonds in two of the most important cell signalling molecules, cyclic-adenosine monophosphates (cAMPs) and cyclic-guanosine monophosphates (cGMPs). PDEs are essential for the inactivation of cAMP and or cGMP by controlling the rate at which they are degraded to regulate their intracellular concentrations and maintain cellular homeostasis [1]. PDEs directly modulate these messenger molecules by degrading cAMP or cGMP when concentrations are elevated beyond the cellular threshold [2]. These enzymes are involved in a wide array of cellular and physiological processes such as metabolism, some immune responses, reproduction, inflammation, regulation of photoreceptors, quorum sensing in prokaryotes, enabling parasite transition through life cycles stages and the list goes on [3].
Contents |
Function
is one of two cyclic nucleotide phosphodiesterases (the other is TbrPDEB2) that have been validated as promising drug targets for the treatment of Human African Trypanosomiasis (HAT), a life-threatening infectious disease also known as sleeping sickness which is caused by Trypanosoma brucei parasites [4]. PDEB1 is a cAMP-specific hydrolase that catalyses the hydrolysis of cAMP to AMP in the catalytic domain of the enzyme. This enzyme has been reported to play a major role in facilitating Trypanosoma motility in host cells and subsequent progression through the parasite life cycle [5].
PDEs can either be cAMP, cGMP or dual-to cAMP and cGMP- specific, and the substrate specificity for the enzyme is a very important consideration in determining enzyme-inhibitor selectivity and specificity. That is, some PDE inhibitors may have higher binding affinity to cAMP-PDEs than to cGMP-PDEs, or similar binding interactions to dual substrate-specific enzymes [6].
Relevance
Trypanosomiasis, or sleeping sickness, is a neglected tropical disease that is fatal if left untreated and is one of the major causes of human and livestock mortality in developing countries. This infection also affects about 72% of European and American tourists who visit safari reserves and game parks in endemic regions [7]. The current treatment options for trypanosomiasis are outdated - such as melarsoprol which was first introduced in 1949- and also sub-optimal typically showing high toxicity, particularly in late-stage infections [8]. There is a clear need for safer, more efficacious drugs to treat sleeping sickness which is why creative studies are being performed to validate new drug targets for trypanosomiasis drug development. PDEB1 makes an interesting drug target for HAT drug discovery due to its essential functions that contribute to parasite fitness and survival.
Structural highlights
|
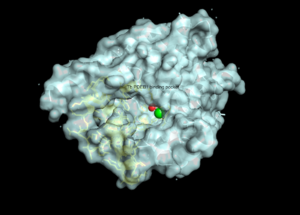
There are several resolved crystal structures for TbPDEB1 in both apo and holo forms which facilitate virtual screenings of libraries of compounds for inhibitory activity against TbPDEB1. The active site for TbPDEB1 contains Magnesium (Mg) and Zinc (Zn) ions with each metal ion forming an octahedral geometry. The metal ion coordinates side chains of two histidine residues and two aspartic residues and ion coordinates D710 and five water molecules.[9]. Only the catalytic domain of the TbPDEB1 enzyme performs hydrolysis of cAMP to AMP [10]. The solved crystal structure of TbPDEB1 enabled the visualization of enzyme ligand and metal binding pocket, which creates a platform for virtual drug screening against the target. That is, compounds can be rapidly selected based on their ability to fit within the binding pocket, and improvements can be made to compounds to support tighter binding.
The resolved crystal structure for the catalytic domain of TbPDEB1 (PDB identifier: 4I15) confirmed the existence of a T. brucei specific P-pocket, a new and exciting direction for improving drug selectivity for T. brucei phosphodiesterase. Following the identification of the parasite specific P-pocket just adjacent to the enzyme binding pocket, new TbPDEB1 inhibitor analogues were created to extend into the parasite enzyme p-pocket. This resulted in improved selectivity for T. brucei PDEB1 over human PDE4, another cAMP specific phosphodiesterase.
Citations
- ↑ Baker DA. Cyclic nucleotide signalling in malaria parasites. Cell Microbiol. 2011 Mar;13(3):331-9. doi: 10.1111/j.1462-5822.2010.01561.x. Epub, 2010 Dec 28. PMID:21176056 doi:http://dx.doi.org/10.1111/j.1462-5822.2010.01561.x
- ↑ Blaazer AR, Singh AK, de Heuvel E, Edink E, Orrling KM, Veerman JJN, van den Bergh T, Jansen C, Balasubramaniam E, Mooij WJ, Custers H, Sijm M, Tagoe DNA, Kalejaiye TD, Munday JC, Tenor H, Matheeussen A, Wijtmans M, Siderius M, de Graaf C, Maes L, de Koning HP, Bailey DS, Sterk GJ, de Esch IJP, Brown DG, Leurs R. Targeting a Subpocket in Trypanosoma brucei Phosphodiesterase B1 (TbrPDEB1) Enables the Structure-Based Discovery of Selective Inhibitors with Trypanocidal Activity. J Med Chem. 2018 May 1. doi: 10.1021/acs.jmedchem.7b01670. PMID:29672041 doi:http://dx.doi.org/10.1021/acs.jmedchem.7b01670
- ↑ Zoraghi R, Seebeck T. The cAMP-specific phosphodiesterase TbPDE2C is an essential enzyme in bloodstream form Trypanosoma brucei. Proc Natl Acad Sci U S A. 2002 Apr 2;99(7):4343-8. PMID:11930001 doi:http://dx.doi.org/10.1073/pnas.062716599
- ↑ Jansen C, Wang H, Kooistra AJ, de Graaf C, Orrling KM, Tenor H, Seebeck T, Bailey D, de Esch IJ, Ke H, Leurs R. Discovery of Novel Trypanosoma brucei Phosphodiesterase B1 Inhibitors by Virtual Screening against the Unliganded TbrPDEB1 Crystal Structure. J Med Chem. 2013 Mar 1. PMID:23409953 doi:http://dx.doi.org/10.1021/jm3017877
- ↑ Shaw S, DeMarco SF, Rehmann R, Wenzler T, Florini F, Roditi I, Hill KL. Flagellar cAMP signaling controls trypanosome progression through host tissues. Nat Commun. 2019 Feb 18;10(1):803. doi: 10.1038/s41467-019-08696-y. PMID:30778051 doi:http://dx.doi.org/10.1038/s41467-019-08696-y
- ↑ Baker DA. Cyclic nucleotide signalling in malaria parasites. Cell Microbiol. 2011 Mar;13(3):331-9. doi: 10.1111/j.1462-5822.2010.01561.x. Epub, 2010 Dec 28. PMID:21176056 doi:http://dx.doi.org/10.1111/j.1462-5822.2010.01561.x
- ↑ Kennedy PGE, Rodgers J. Clinical and Neuropathogenetic Aspects of Human African Trypanosomiasis. Front Immunol. 2019 Jan 25;10:39. doi: 10.3389/fimmu.2019.00039. eCollection, 2019. PMID:30740102 doi:http://dx.doi.org/10.3389/fimmu.2019.00039
- ↑ Kennedy PG. Human African trypanosomiasis of the CNS: current issues and challenges. J Clin Invest. 2004 Feb;113(4):496-504. doi: 10.1172/JCI21052. PMID:14966556 doi:http://dx.doi.org/10.1172/JCI21052
- ↑ Jansen C, Wang H, Kooistra AJ, de Graaf C, Orrling KM, Tenor H, Seebeck T, Bailey D, de Esch IJ, Ke H, Leurs R. Discovery of Novel Trypanosoma brucei Phosphodiesterase B1 Inhibitors by Virtual Screening against the Unliganded TbrPDEB1 Crystal Structure. J Med Chem. 2013 Mar 1. PMID:23409953 doi:http://dx.doi.org/10.1021/jm3017877
- ↑ Kunz S, Luginbuehl E, Seebeck T. Gene conversion transfers the GAF-A domain of phosphodiesterase TbrPDEB1 to one allele of TbrPDEB2 of Trypanosoma brucei. PLoS Negl Trop Dis. 2009 Jun 9;3(6):e455. doi: 10.1371/journal.pntd.0000455. PMID:19513125 doi:http://dx.doi.org/10.1371/journal.pntd.0000455