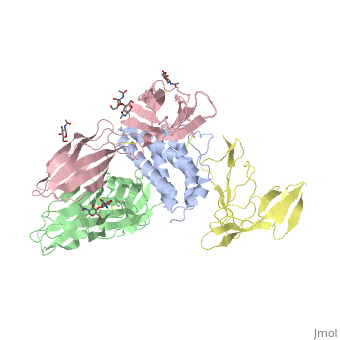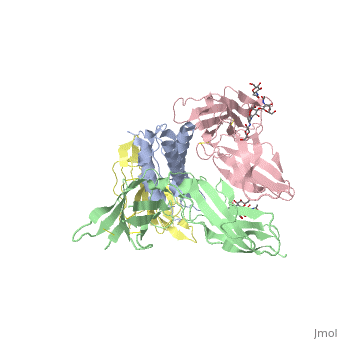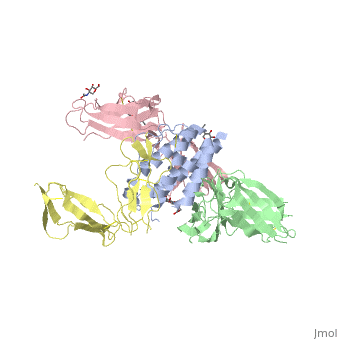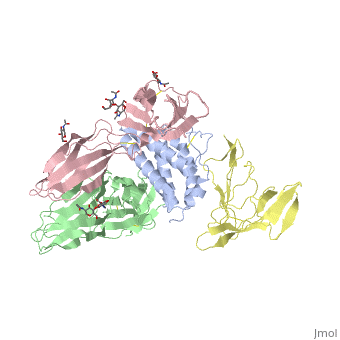Interleukin receptor
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<StructureSection load='2b5i' size='340' side='right' caption='Glycosylated interleukin-2 receptor α (yellow), β (green), γ (pink) chains complex with interleukin-2 (grey), [[2b5i]]' scene=''> | <StructureSection load='2b5i' size='340' side='right' caption='Glycosylated interleukin-2 receptor α (yellow), β (green), γ (pink) chains complex with interleukin-2 (grey), [[2b5i]]' scene=''> | ||
'''Interleukin receptors''' (ILR) are cytokine receptor which are classified into type I, type II and others. '''ILR type I''' are cell surface receptors which recognize cytokines with four α-helical strands. '''ILR type II''' are similar to type I but lack the former’s signature sequence: WXSWS. Interleukin-1 receptor belongs to the ILR of immunoglobulin family. IL1R contains two types. Type I (IL1R1) binds interleukin-1 (IL1) transmitting its inflammatory effect. Interleukin-1 receptor type II (IL1R2) suppresses IL1 activity. Another suppressor of IL1R activity is the interleukin-1 receptor antagonist (IL1R1A). | '''Interleukin receptors''' (ILR) are cytokine receptor which are classified into type I, type II and others. '''ILR type I''' are cell surface receptors which recognize cytokines with four α-helical strands. '''ILR type II''' are similar to type I but lack the former’s signature sequence: WXSWS. Interleukin-1 receptor belongs to the ILR of immunoglobulin family. IL1R contains two types. Type I (IL1R1) binds interleukin-1 (IL1) transmitting its inflammatory effect. Interleukin-1 receptor type II (IL1R2) suppresses IL1 activity. Another suppressor of IL1R activity is the interleukin-1 receptor antagonist (IL1R1A). | ||
| + | |||
| + | '''TYPE-1 INTERLEUKIN-1 RECEPTOR COMPLEXED WITH INTERLEUKIN-1 BETA''' | ||
| + | |||
| + | [http://en.wikipedia.org/wiki/Interleukin-1_receptor Interleukin-1 receptor] complex with ligand and go through the plasma membrane. <scene name='57/571319/Scene_2/2'> Type 1 Interleukin-1 receptor complex with Interleukin-1 beta</scene> 3D structure is showing here. Ribbon diagram of s-IL 1R complex to IL-1β. The complex has approximate dimensions of 97Å × 52Å ×35Å with one s-IL1R molecule wrapping around the IL-1β molecule with 1:1 ratio. In the <scene name='57/571319/Scene_2/3'>complex</scene>, domain 3 provides a 'lid' which covers most of the top of the IL-1β β-barrel, whereas domains 1 and 2 from a groove which binds to the lower rim of the barrel. Here, Domains 1,2 and 3 of s-IL 1R are colored light, medium and dark blue, respectively. IL-1β is yellow, with site A residues in green and site B residues in red. The structure is oriented so that the carboxy terminus of s-IL 1R and the cell membrane are at the bottom of the picture. | ||
| + | |||
| + | '''STRUCTURE OF THE INTERLEUKIN-1BETA SIGNALING COMPLEX''' | ||
| + | |||
| + | [[Image:Ternary complex paradigm.png||250px|right|]] | ||
| + | |||
| + | <scene name='57/571319/Scene_3/1'> Interleukin-1 beta signal complex</scene> 3D structure was showed here, and the cartoon (right) showed the formation of the ternary complex paradigm, the primary receptors ('''IL-1R''') first bind their corresponding cytokine ligands ('''IL-1β''') and then engage the accessory receptor ('''IL-1RAcP''') (which is incapable of binding cytokines by itself). The overall ternary architecture of the IL-1β–IL-1RI–IL-1RAcP signaling complex has the cytoplasmic TIR domain necessary for signal transduction. The IL-1RI-IL-1RAcP interface was most hydrogen-bonded and signal was transferred through the highly packed hydrophobic region between receptor accessory and IL-1R liganded with IL-1β. | ||
| + | |||
| + | The dimer complex with conserved area was showed when you <scene name='57/571319/Scene_3/2'>click here</scene>. The insert are ligands (IL-1β), which are present as conserved structure as well as green ribbon. The two IL-1Rs and two IL-1RAcPs are colored as purple and blue, respectively. | ||
| + | |||
| + | ==Clinical significance== | ||
| + | Why are people interested in IL-1? Because IL-1 cytokines family are usually over-expressed at tumor sites or inflammatory, these cytokines could be used as bio-markers to help diagnose in advance. Also, since IL-1α, IL-1β and IL-1ra all have the ability to bind to the type 1 IL-1 receptor (IL-1R), and the binding of IL-1α or IL-1β to IL-1R is an early step in IL-1 signal transduction, blocking this interaction may therefore be a useful target for the development of new drugs. | ||
See also: | See also: | ||
Revision as of 11:55, 1 May 2022
| |||||||||||