User:Isabelle Kressy/Sandbox 1
From Proteopedia
| Line 2: | Line 2: | ||
<StructureSection load='3rad' size='340' frame='true' align='right' caption='Type IV topoisomerase DNA cleavage complex with Quinolone. PDB ID: 3RAD'/> | <StructureSection load='3rad' size='340' frame='true' align='right' caption='Type IV topoisomerase DNA cleavage complex with Quinolone. PDB ID: 3RAD'/> | ||
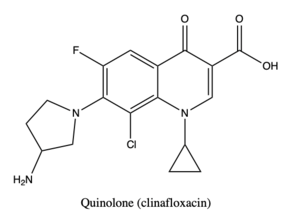
| - | Topoisomerase IV in S. pneumoniae is a paralogue of type II topoisomerase. This structure is a complex of topoisomerase, DNA, and <scene name='90/909964/Quinolone_molecule/1'>Quinolone</scene>, a drug that targets type II topoisomerases in Gram-negative and Gram-positive bacteria <ref>PMID:27655731</ref>. Topoisomerase IV consists of two subunits that function to regulate supercoiling and disentangle DNA. | + | Topoisomerase IV in S. pneumoniae is a paralogue of type II topoisomerase. This structure is a complex of topoisomerase, DNA, and <scene name='90/909964/Quinolone_molecule/1'>Quinolone</scene>, a drug that targets type II topoisomerases in Gram-negative and Gram-positive bacteria <ref>PMID:27655731</ref>. Topoisomerase IV consists of two subunits that function to regulate supercoiling and disentangle DNA <ref>PMID:16023670</ref>. |
== Function == | == Function == | ||
The main function of topo IV in bacteria is to disentangle and unknot DNA in order to unlink chromosomes <ref>PMID:16023670</ref>. Topo IV is generally located at DNA replication forks to relax [https://en.wikipedia.org/wiki/DNA_supercoil positive supercoiled] DNA and to work with chromosome segregation machinery. | The main function of topo IV in bacteria is to disentangle and unknot DNA in order to unlink chromosomes <ref>PMID:16023670</ref>. Topo IV is generally located at DNA replication forks to relax [https://en.wikipedia.org/wiki/DNA_supercoil positive supercoiled] DNA and to work with chromosome segregation machinery. | ||
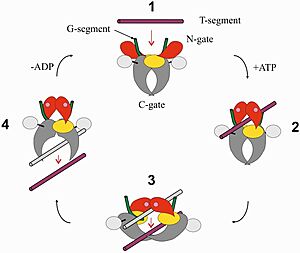
| - | In general, type II topoisomerases undergo a strand-passage mechanism to disentangle chromosomes. During replication, positive supercoils are created ahead of the replication fork and [https://www.jbc.org/article/S0021-9258(18)80886-9/fulltext precatenanes] behind the fork. Topo IV removes positive supercoils, catenanes, and knots so that replication can occur. Its mechanism occurs through a cycle, beginning when one DNA duplex, the “gate” or G-segment, binds to the cleavage complex. When the ATPase domains bind to ATP, they dimerize and capture a second DNA duplex, the “transfer” or T-segment. This causes a double-strand break in the G-segment and subsequent passage of the T-segment through the gap (termed the DNA-gate) opened by the G-segment. The G-segment is then resealed and both DNA duplexes are released from the enzyme. | + | In general, type II topoisomerases undergo a strand-passage mechanism to disentangle chromosomes. During replication, positive supercoils are created ahead of the replication fork and [https://www.jbc.org/article/S0021-9258(18)80886-9/fulltext precatenanes] behind the fork <ref>PMID:16023670</ref>. Topo IV removes positive supercoils, catenanes, and knots so that replication can occur. Its mechanism occurs through a cycle, beginning when one DNA duplex, the “gate” or G-segment, binds to the cleavage complex. When the ATPase domains bind to ATP, they dimerize and capture a second DNA duplex, the “transfer” or T-segment. This causes a double-strand break in the G-segment and subsequent passage of the T-segment through the gap (termed the DNA-gate) opened by the G-segment. The G-segment is then resealed and both DNA duplexes are released from the enzyme <ref>PMID:16023670</ref>. |
[[Image:Topo_IV_mechanism.jpg|300px|right|thumb| Strand-passage mechanism of topo IV]] | [[Image:Topo_IV_mechanism.jpg|300px|right|thumb| Strand-passage mechanism of topo IV]] | ||
Revision as of 02:55, 5 May 2022
Contents |
Quinolone(Clinafloxacin)-DNA cleavage complex of type IV topoisomerase from S. pneumoniae
|
Topoisomerase IV in S. pneumoniae is a paralogue of type II topoisomerase. This structure is a complex of topoisomerase, DNA, and , a drug that targets type II topoisomerases in Gram-negative and Gram-positive bacteria [1]. Topoisomerase IV consists of two subunits that function to regulate supercoiling and disentangle DNA [2].
Function
The main function of topo IV in bacteria is to disentangle and unknot DNA in order to unlink chromosomes [3]. Topo IV is generally located at DNA replication forks to relax positive supercoiled DNA and to work with chromosome segregation machinery.
In general, type II topoisomerases undergo a strand-passage mechanism to disentangle chromosomes. During replication, positive supercoils are created ahead of the replication fork and precatenanes behind the fork [4]. Topo IV removes positive supercoils, catenanes, and knots so that replication can occur. Its mechanism occurs through a cycle, beginning when one DNA duplex, the “gate” or G-segment, binds to the cleavage complex. When the ATPase domains bind to ATP, they dimerize and capture a second DNA duplex, the “transfer” or T-segment. This causes a double-strand break in the G-segment and subsequent passage of the T-segment through the gap (termed the DNA-gate) opened by the G-segment. The G-segment is then resealed and both DNA duplexes are released from the enzyme [5].
Structural Description
The active site is a tetramer made up of and subunits. The ParC subunit contains an , which is linked to the C-terminal domain (CTD) that forms a β-pinwheel shape and functions for DNA binding. This domain is described as a closed dimer with a charge DNA-binding domain. This favors the passage of DNA and DNA unlinking from the complex. In contrast, the N-terminal of the ParE subunits forms the . Additionally, ParE has a C-terminal Mg2+ binding domain that consists of a (residues E433, D506, D508). The C-gate consists of the two ParC subunits and the G-gate consists of the two ParE subunits.
Structural Features Relating to Function
Upon binding the ATPase domains, the G-segment distorts from linear B form to bent and extended A form DNA. The T-segment approaches and is “captured” by the ATPase domains. When the T-segment is “captured”, it moves to the middle of the two ATPase domains. Topo IV intercalates the T-segment into its major groove to provide binding stability. Once the two DNA duplexes are in place, the ATPase domains use ATP to cause cross interpolation of the first 20 residues of ParE and dimerizing the two ATPase domains. This causes DNA cleavage in the G-segment by both ParC and ParE. (Y118 on ParC) bind to 5’ phosphates on the G-segment to cause a double-stranded break. This, paired with the torsional stress on the G-segment, mediate the cleavage. When the DNA-gate opens to let the T-segment through, the ATPase domains rotate the T-segment by 45˚ and simultaneously moves alpha helices from the ParE subunits out of the way for the T-segment to pass. Once the T-segment is next to the C-gate, the G-gate closes and reseals the G-segment, which causes T-segment release by the opening and closing of the C-gate. Closure of the G-gate is caused by the ATPase domains rotating to its original position, inducing ATP hydrolysis, and releasing ADP and the G-segment.
Quinolone
Quinolone targets type II topoisomerases and is a very important drug for microbial infections as bacteria are gaining resistance to β-lactam antibiotics. Although its resistance is less than β-lactam antibiotics, there is still a need to produce new drugs of this kind to bypass resistance mutations. Quinolones prevent the strand passage of the T-segment through the N-gate by trapping the covalent enzyme-DNA complex to prevent DNA resealing and causing apoptosis. Quinolone interacts with magnesium and three key residues, in the topo IV active site and decreases DNA replication.
Evolutionarily Related Proteins
|
Topo IV works closely with gyrase, another type II topoisomerase, to uncoil DNA and ensure that replication can take place. Its main function is to relax positive supercoils and catalyze the formation of negative supercoils. Unlike topo IV, gyrase cannot unknot and decatenate DNA. Structurally, these two topoisomerases are very similar. One study found that the E. coli CTD of the ParC subunit is a degenerate form of the CTD in gyrase and that it is positioned differently for substrate specificity.
Other related proteins are listed below. 1S14 2INR
Related Structures
2NOV 3FOE 3FOF 3K9F 3KSA 3KSB 3LTN 3RAE 3RAF
</StructureSection>
References
- ↑ Laponogov I, Pan XS, Veselkov DA, Cirz RT, Wagman A, Moser HE, Fisher LM, Sanderson MR. Exploring the active site of the Streptococcus pneumoniae topoisomerase IV-DNA cleavage complex with novel 7,8-bridged fluoroquinolones. Open Biol. 2016 Sep;6(9). pii: rsob.160157. doi: 10.1098/rsob.160157. PMID:27655731 doi:http://dx.doi.org/10.1098/rsob.160157
- ↑ Corbett KD, Schoeffler AJ, Thomsen ND, Berger JM. The structural basis for substrate specificity in DNA topoisomerase IV. J Mol Biol. 2005 Aug 19;351(3):545-61. PMID:16023670 doi:10.1016/j.jmb.2005.06.029
- ↑ Corbett KD, Schoeffler AJ, Thomsen ND, Berger JM. The structural basis for substrate specificity in DNA topoisomerase IV. J Mol Biol. 2005 Aug 19;351(3):545-61. PMID:16023670 doi:10.1016/j.jmb.2005.06.029
- ↑ Corbett KD, Schoeffler AJ, Thomsen ND, Berger JM. The structural basis for substrate specificity in DNA topoisomerase IV. J Mol Biol. 2005 Aug 19;351(3):545-61. PMID:16023670 doi:10.1016/j.jmb.2005.06.029
- ↑ Corbett KD, Schoeffler AJ, Thomsen ND, Berger JM. The structural basis for substrate specificity in DNA topoisomerase IV. J Mol Biol. 2005 Aug 19;351(3):545-61. PMID:16023670 doi:10.1016/j.jmb.2005.06.029