BASIL2022GV3HDT
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
| - | == ''In | + | == ''In Silico'' Anaylsis == |
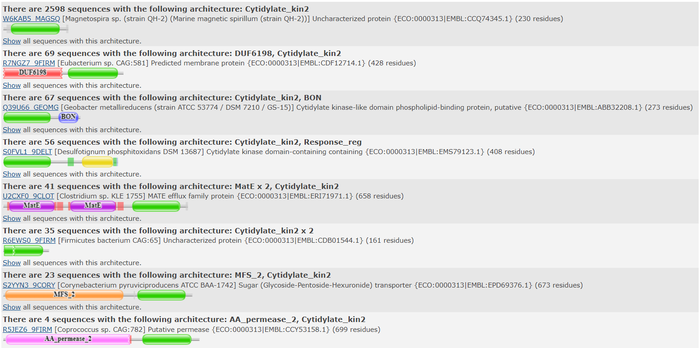
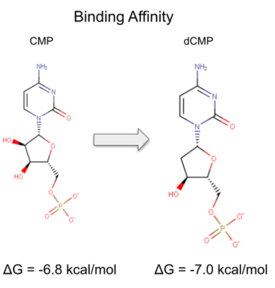
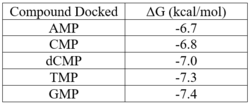
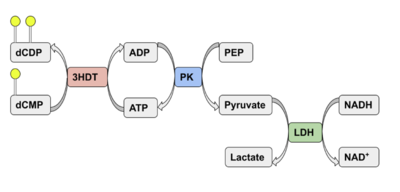
We used a variety of ''in silico'' tools to find similarities between 3HDT and other amino acid sequences, protein families, and 3-dimensional structures of known proteins in the PDB. Below are the recorded results and information from each database. From this information, a function was hypothesized for 3HDT and potential substrates such as dCMP were selected for kinase assays. | We used a variety of ''in silico'' tools to find similarities between 3HDT and other amino acid sequences, protein families, and 3-dimensional structures of known proteins in the PDB. Below are the recorded results and information from each database. From this information, a function was hypothesized for 3HDT and potential substrates such as dCMP were selected for kinase assays. | ||
| Line 95: | Line 95: | ||
| - | ===='''Coupled | + | ===='''Coupled Kinase Assay'''==== |
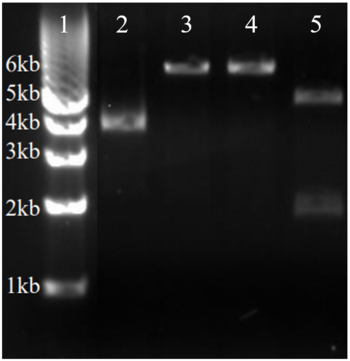
Two rounds of coupled kinase assays were run using 3HDT with ATP and dCMP as substrates. Various concentrations of dCMP between 5-10mM were used in a total of 8 assays. In the first round of assays (3 assays) 45.6ng of 3HDT and increasing concentrations of 5.0, 7.5, and 10.0mM of dCMP were added to wells each containing 100μL total. A concentration of 7.5mM dCMP resulted in the highest specific activity (0.377 U/mg) while increasing the substrate concentration to 10mM slightly lowered specific activity to 0.348 U/mg. When we repeated the first three kinase assays (5.0, 7.5, 10.0mM dCMP), there were discrepancies in the results indicating a potential experimental error such improper mixing, bubbles being present in the well, or too much time passing between adding substrate to the well and measuring absorbance in the plate reader. Additionally, the protein in the second round of assays was older (original protein but approximately a week had passed from time of over-expression) which could have affected the specific activity in those trials due to the protein starting to expire/decrease in function. | Two rounds of coupled kinase assays were run using 3HDT with ATP and dCMP as substrates. Various concentrations of dCMP between 5-10mM were used in a total of 8 assays. In the first round of assays (3 assays) 45.6ng of 3HDT and increasing concentrations of 5.0, 7.5, and 10.0mM of dCMP were added to wells each containing 100μL total. A concentration of 7.5mM dCMP resulted in the highest specific activity (0.377 U/mg) while increasing the substrate concentration to 10mM slightly lowered specific activity to 0.348 U/mg. When we repeated the first three kinase assays (5.0, 7.5, 10.0mM dCMP), there were discrepancies in the results indicating a potential experimental error such improper mixing, bubbles being present in the well, or too much time passing between adding substrate to the well and measuring absorbance in the plate reader. Additionally, the protein in the second round of assays was older (original protein but approximately a week had passed from time of over-expression) which could have affected the specific activity in those trials due to the protein starting to expire/decrease in function. | ||
| Line 117: | Line 117: | ||
== Conclusion & Future Experiments == | == Conclusion & Future Experiments == | ||
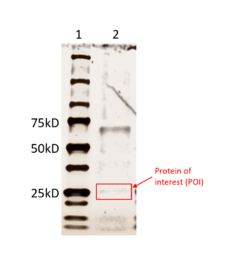
| - | Our protein, 3HDT, was confirmed to be present using SDS-PAGE and showed activity during coupled kinase assays. While this confirms that 3HDT is a kinase, the true substrate was likely not dCMP due to the low specific activity values. Further research should be done with molecules such as TMP and GMP in the future to narrow down potential nucleotide substrates or elucidate other types of compounds to be considered as ligands for 3HDT. Additionally, in the future we would like to | + | Our protein, 3HDT, was confirmed to be present using SDS-PAGE and showed activity during coupled kinase assays. While this confirms that 3HDT is a kinase, the true substrate was likely not dCMP due to the low specific activity values. Further research should be done with molecules such as TMP and GMP in the future to narrow down potential nucleotide substrates or elucidate other types of compounds to be considered as ligands for 3HDT. Additionally, in the future we would like to improve upon our protein purification process to try and get a higher protein concentration than what we achieved as the low concentration may have affected our coupled kinase assay results. |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 20:03, 7 May 2022
Characterizing Putative Kinase 3HDT and its Potential Substrates
| |||||||||||
References
- ↑ National Center for Biotechnology Information (NCBI)[Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [1988] – [cited 2022 April 23].
- ↑ Pfam: The protein families database in 2021: J. Mistry, S. Chuguransky, L. Williams, M. Qureshi, G.A. Salazar, E.L.L. Sonnhammer, S.C.E. Tosatto, L. Paladin, S. Raj, L.J. Richardson, R.D. Finn, A. Bateman Nucleic Acids Research (2020) doi: 10.1093/nar/gkaa913
- ↑ Holm L (2020) Using Dali for protein structure comparison. Methods Mol. Biol. 2112, 29-42.
- ↑ J. Yu, Y. Zhou, I. Tanaka, M. Yao, Roll: A new algorithm for the detection of protein pockets and cavities with a rolling probe sphere. Bioinformatics, 26(1), 46-52, (2010) [PMID: 19846440]
- ↑ Small-Molecule Library Screening by Docking with PyRx. Dallakyan S, Olson AJ. Methods Mol Biol. 2015;1263:243-50.
- ↑ The PyMOL Molecular Graphics System, Version 1.7.4.5 Edu Schrödinger, LLC.
- ↑ Wallace, A C et al. “LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions.” Protein engineering vol. 8,2 (1995): 127-34. doi:10.1093/protein/8.2.127
Proteopedia Page Contributors and Editors (what is this?)
Jesse D. Rothfus, Autumn Forrester, Bonnie Hall, Jaime Prilusky