Structure of mammalian plasma fetuin-B and its mechanism of selective metallopeptidase inhibition
Anna Cuppari, Hagen Körschgen, Dirk Fahrenkamp, Carlo Schmitz, Tibisay Guevara, Konstantin Karmilin, Michael Kuske, Mario Olf, Eileen Dietzel, Irene Yiallouros, Daniele de Sanctis, Theodoros Goulas, Ralf Weiskirchen, Willi Jahnen-Dechent, Julia Floehr, Walter Stöcker, Luca Jovine and F. Xavier Gomis-Rüth [1]
Molecular Tour
Mammalian fertility is critically dependent on the zona pellucida (ZP), a glycoprotein matrix surrounding the oocyte that acts as a gatekeeper for gamete fusion. Before fertilization ZP is kept permeable to sperm but after fertilization it rapidly hardens to prevent other sperm from penetrating the oocyte. These transactions are tightly regulated by a metallopeptidase:inhibitor tandem consisting of ovastacin and fetuin-B, respectively. We characterized the very tight and specific interaction of fetuin-B and ovastacin at the molecular level and the complex of the inhibitor with the close ortholog astacin at the structural level. The results have unveiled the "raised-elephant-trunk" mechanism of inhibition, which is novel for metallopeptidases and whose suppression would pave the way to the design of new anticonceptives.
. Domain CY1 is in orange, the linker (LNK) in red, CY2 in turquoise and the CTR in purple. N-glycans are shown as sticks and labeled with an "A" and "B", P155 and D156 from the CPDCP-trunk are shown for their side chains and labeled. Disulfides are shown as yellow lines. The structure of fetuin-B (residues R29-P388) within its astacin complex includes domains and segments , linker , (S159-E267, except loops T218-Q227 and L246-L251), and S268-V272 plus P303-P388 from the (S268-P388). The N-terminal 118-residue exhibits the cystatin fold and consists of a twisted and curled five-stranded antiparallel β-sheet (strands β1-β5) of simple up-and-down connectivity. An α-helix (α1) is inserted between β1 and β2 through short linkers and nestles perpendicularly to the strands in the concave face of the sheet. Strand pairs β2/β3 and β4/β5 are connected by short loops and arranged as hairpins I and II, respectively. In contrast, β3 and β4 are connected by a long 21-residue loop (Lβ3β4), which was found in several conformations in ovocystatin and was involved in inhibition of legumain. This “legumain-binding loop” is internally crosslinked by disulfide C96-C107. A second disulfide staples β4 and β5 together, roughly at half strand length. A fifth cysteine (C39) in Lβ1α1 is bound to the CTR through a disulfide, and , respectively. The C-terminal strand of CY1 (β5) contains a bulge and leads to the , which consists of a short α-helix (α2) and segment C154-P-D-C-P158 arranged in a tight 1,4-turn of type I, hereafter referred to as “CPDCP-trunk”. Owing to the prolines and the cysteines, which are disulfide-linked (C154-C157), this structure forms a rigid 14-atom ring that protrudes from the molecular surface. The LNK is followed by the , which likewise adopts the cystatin fold and is topologically equivalent to CY1. It interacts through the convex face of its β-sheet with Lα1β2 and the legumain-binding loop from CY1. After the C-terminal strand of CY2 (β10), the polypeptide chain of fetuin-B enters the , which is generally irregularly folded, disordered between P273 and A302, and cleaved at S296-S297. After P303, the chain runs parallel to strand β5 of CY1 as strand β11, thus providing a first anchor to CY1 for the downstream part of the domain. The remaining 78 residues of the CTR could be traced but are flexible: they lack a regular secondary structure and run irregularly along the convex surface of the CY1 β-sheet. Only two short β-strands (β12 and β13), which coincide with secondary-structure predictions for the CTR, interact with each other in an antiparallel manner. Toward the domain C-terminus, C374 is disulfide-linked to C39, in this way providing a second anchor between the CTR and CY1. It is noteworthy that is conserved within fetuins, kininogens, and histidine-rich glycoproteins. This suggests a similar link between CY1 and CTR in other fetuins, as already experimentally proven for human fetuin-A and human kininogen.
(in tan). of displaying selected residues participating in the interaction between fetuin-B (Cα-ribbon in plum; from left to right, segments S146-D162, M111-E114, N197-Y206, and T241-S245, carbons in pink) and astacin (Cα-ribbon in tan, carbons in orange, catalytic zinc as a magenta sphere). Astacin segments participating in the interaction include S62-V68 from the upper-rim strand and a preceding bulge above the primed side of the cleft; Q76 from the fifth strand; G83-C84 from the loop connecting the fifth sheet strand with the active-site helix plus disulfide C64-C84; the zinc-binding site; and Y101, D121-D129, F154-W158, and Y177 from the LSD. Fetuin-B segments involved include M111-Y113 from the legumain-binding loop of CY1, K147-S159 from the LNK and CY2 strand β6, N198-Y206 from CY2 hairpin I and, to a lesser extent, S245 plus Q253-V255 from CY2 hairpin II, whose tip is disordered.
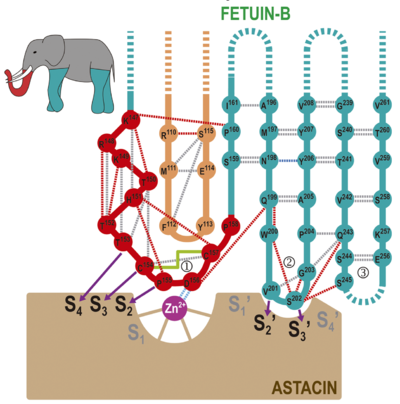
Scheme of the proposed “raised elephant-trunk model”. The CPDCP-trunk (1) would be the raised trunk (in red) and hairpin I (2) and II (3) the two front and back limbs (in turquoise), respectively (see top-left inset). The trunk contacts the catalytic zinc ion (magenta sphere) of astacin (in brown) by residues nestling into cleft sub-sites
S4, S3, S2, S2’, and S3’, as shown by purple arrows. Dashed lines stand for distantly connected segments or disordered loops. Hydrogen bonds between main-chain atoms, between side- and main-chain atoms, and between side chains are shown as grey, red and blue dashed lines, respectively. The electrostatic interaction between the catalytic zinc and D156 is in light blue. The disulfide connecting the CPDCP-trunk is shown as a green solid line.
The inhibitory mechanism of cysteine peptidases through monomeric cystatins was dubbed the “elephant-trunk model” and is mainly exerted by the N-terminal segment of the inhibitor (“trunk”; see the static image above). This segment precedes the first strand of the β-sheet and occupies S1 and non-primed upstream sub-sites of the cleft. On the primed side, hairpin I contains the conserved sequence QXVXG, which functions similarly to fetuin-B hairpin I with motif QWVXGP. Finally, cystatin hairpin II, provides a scaffold for hairpin I and performs ancillary interactions with the enzyme through conserved motif PW. Overall, the inhibitory mechanism of MPs through fetuin-B, in which the extended N-terminal trunk is replaced with a compact CPDCP-trunk that resembles a “raising trunk”, contains elements of similarity with the hydrophobic tripartite wedge of cystatins tackling cysteine peptidases. This suggests that gene duplication and molecular evolution of cysteine-peptidase targeting cystatins eventually led to inhibition of peptidases of a different chemical class. We thus hereby propose the term “raised-elephant-trunk mechanism” to describe the modus inhibendi of fetuin-B against astacins.
PDB references: mouse fetuin-B, 6hpv; complex with astacin, 6ht9.
References
- ↑ Cuppari A, Korschgen H, Fahrenkamp D, Schmitz C, Guevara T, Karmilin K, Kuske M, Olf M, Dietzel E, Yiallouros I, de Sanctis D, Goulas T, Weiskirchen R, Jahnen-Dechent W, Floehr J, Stoecker W, Jovine L, Gomis-Ruth FX. Structure of mammalian plasma fetuin-B and its mechanism of selective metallopeptidase inhibition. IUCrJ. 2019 Feb 28;6(Pt 2):317-330. doi: 10.1107/S2052252519001568. eCollection, 2019 Mar 1. PMID:30867929 doi:http://dx.doi.org/10.1107/S2052252519001568



