User:Clara Costa D'Elia/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
Photosynthesis represents the main source of energy sustaining life on earth. The primary process of photosynthesis starts with the absorption of sunlight by an arrangement of photosynthetic pigments embedded into a proteic matrix called the light harvesting (LH) antenna complexes. The excitation energy of | Photosynthesis represents the main source of energy sustaining life on earth. The primary process of photosynthesis starts with the absorption of sunlight by an arrangement of photosynthetic pigments embedded into a proteic matrix called the light harvesting (LH) antenna complexes. The excitation energy of | ||
photosynthetic pigments is then transferred to the photosynthetic reaction center where it is converted into chemical energy. The primary process of photosynthesis occurs with a quantum yield close to unity. | photosynthetic pigments is then transferred to the photosynthetic reaction center where it is converted into chemical energy. The primary process of photosynthesis occurs with a quantum yield close to unity. | ||
| - | Most photosynthetics pigments are chlorophylls (Chl), bacteriochlorophylls (BChl), and carotenoids, they represent the keystone for energy storage in photosynthetic organisms. | + | Most photosynthetics pigments are chlorophylls (Chl), bacteriochlorophylls (BChl), and carotenoids, they represent the keystone for energy storage in photosynthetic organisms.<ref> https://doi.org/10.1021/jp203826q </ref> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
== Function == | == Function == | ||
| Line 43: | Line 32: | ||
<scene name='91/911263/Water_molecules_on_the_lhc_ii/1'>Water molecules</scene> | <scene name='91/911263/Water_molecules_on_the_lhc_ii/1'>Water molecules</scene> | ||
<scene name='91/911263/Side_chains_of_the_lhc_ii/1'>Side chains</scene> | <scene name='91/911263/Side_chains_of_the_lhc_ii/1'>Side chains</scene> | ||
| + | |||
| + | ==About the pigments== | ||
| + | The BChl a Qy transition dipole moments are responsible for the near infrared absorption by Bchl a molecules at generic wavelengths 800 nm and 850 nm, and for this reason these pigments are called B800 and B850.<ref> https://doi.org/10.1016/S0022-2836(03)00024-X</ref> | ||
| + | |||
| + | Like other dipole moments, the transition dipole refers to a difference in charge from one region of a molecule to another. The transition dipole occurs when an electron is excited from the ground state to an excited state. The charge distribution of the ground state is different from that of the excited state, and it is this change in electron density between the two states that leads to the transitoin dipole. | ||
| + | <ref> https://bmc1.utm.utoronto.ca/~vijay/prototype_V12/physChem/molExcit/p08/index.html</ref> | ||
| + | |||
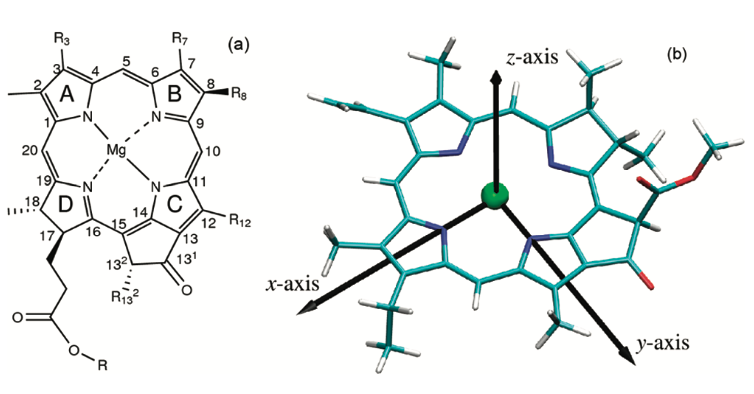
| + | By convention, the y molecular axis of chlorophylls and bacteriochlorophylls is defined as the axis passing through the N atoms of rings A and C; | ||
| + | [[Image:Qy 2.png]]<ref> https://doi.org/10.1021/jp203826q </ref> | ||
| + | |||
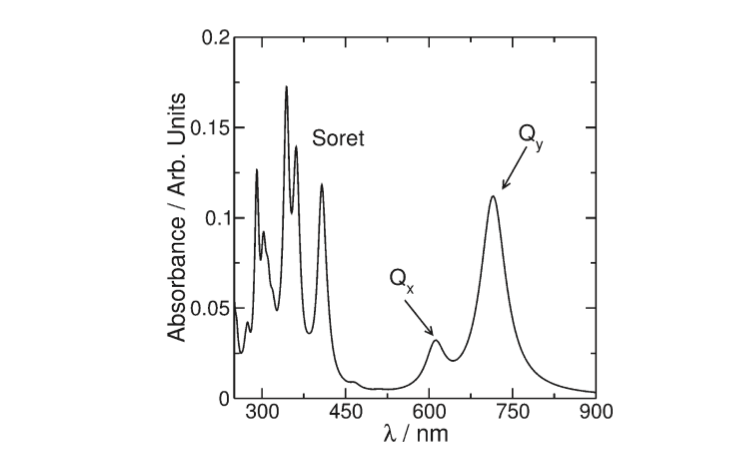
| + | The spectrum of photosynthetic pig- | ||
| + | ments exhibits essentially two characteristic absorption bands | ||
| + | (Figure 2): one of them called the Soret band can be found in the | ||
| + | UV region and is a complex band composed of a large series of | ||
| + | electronic transitions. The other called Q is in the visible region | ||
| + | of the spectrum and is the most important for the photophysics | ||
| + | involved in the photosynthetic process | ||
| + | [[Image:QY_axis.png]]<ref> https://doi.org/10.1021/jp203826q </ref> | ||
| + | |||
| + | Important to understanding how these complexes trap energy is the degree of delocalisation of the lowest energy states between 850 nm and 870 nm.<ref> https://doi.org/10.1016/S0022-2836(03)00024-X</ref> | ||
| + | |||
| + | |||
| + | The delocalization energy is the extra stability a compound has as a result of having delocalized electrons. Electron delocalization is also called resonance. Therefore, delocalization energy is also called resonance energy. The resonance hybrid is more stable than any of its resonance contributors is predicted to be | ||
| + | |||
| + | Charge delocalization is a stabilizing force because it spreads energy over a larger area rather than keeping it confined to a small area. Since electrons are charges, the presence of delocalized electrons brings extra stability to a system compared to a similar system where electrons are localized. The stabilizing effect of charge and electron delocalization is known as resonance energy. | ||
| + | |||
| + | Since conjugation brings up electron delocalization, it follows that the more extensive the conjugated system, the more stable the molecule (i.e. the lower its potential energy). If there are positive or negative charges, they also spread out as a result of resonance. | ||
| + | |||
| + | <ref>https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Valence_Bond_Theory/Delocalization_of_Electrons</ref> | ||
== Relevance == | == Relevance == | ||
Revision as of 21:58, 5 June 2022
Light Harvesting Complex II
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ https://doi.org/10.1021/jp203826q
- ↑ https://www.sciencedirect.com/science/article/pii/S002228360300024X?via%3Dihub
- ↑ https://doi.org/10.1016/S0022-2836(03)00024-X
- ↑ https://bmc1.utm.utoronto.ca/~vijay/prototype_V12/physChem/molExcit/p08/index.html
- ↑ https://doi.org/10.1021/jp203826q
- ↑ https://doi.org/10.1021/jp203826q
- ↑ https://doi.org/10.1016/S0022-2836(03)00024-X
- ↑ https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Valence_Bond_Theory/Delocalization_of_Electrons