User:Clara Costa D'Elia/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
==introduction== | ==introduction== | ||
Photosynthesis represents the main source of energy sustaining life on earth. The primary process of photosynthesis starts with the absorption of sunlight by an arrangement of photosynthetic pigments embedded into a proteic matrix called the light harvesting (LH) antenna complexes. The excitation energy of | Photosynthesis represents the main source of energy sustaining life on earth. The primary process of photosynthesis starts with the absorption of sunlight by an arrangement of photosynthetic pigments embedded into a proteic matrix called the light harvesting (LH) antenna complexes. The excitation energy of | ||
| - | photosynthetic pigments is then transferred to the photosynthetic reaction center where it is converted into chemical energy. | + | photosynthetic pigments is then transferred to the photosynthetic reaction center where it is converted into chemical energy. |
| - | + | .<ref> https://doi.org/10.1021/jp203826q </ref> | |
| + | |||
| + | . Typically, purple bacteria contain two types of antenna complexes, both of which are | ||
| + | integral membrane proteins. LHl, is intimately | ||
| + | associated with the reaction centre forming the so-called 'core' | ||
| + | complex. Arranged more peripherally to this, and present in | ||
| + | variable amounts, is the second type, LH2. Both types of complex are built on a similar modular principle. | ||
| + | In order to increase the spectral cross-section of absorption, purple bacteria also produce light-harvesting complexes. In most cases a primary light-harvesting complex (LH1) and peripheral light-harvesting complexes (LH2) are synthesised | ||
| + | LH2 complexes are produced in variable amounts according to the available light levels, the absorbance range of the particular LH2 (800 and 850, 800 and 820 nm), the temperature, and the bacterial species and strain (Zuber & Brunisholz, 1991). | ||
| + | When purple bacteria are grown under anaerobic conditions they incorporate the photosynthetic apparatus described above into invaginated intracytoplasmic phospholipid membranes.<ref>https://doi.org/10.1038/374517a0</ref> | ||
== Function == | == Function == | ||
| Line 13: | Line 22: | ||
In the LHC The proteins determine the disposition of the pigments, therefore changing and influencing their absorption spectra. | In the LHC The proteins determine the disposition of the pigments, therefore changing and influencing their absorption spectra. | ||
| - | + | The properties and times scales of energy transfer arise from the relative pigment interaction energies and pigment site energy disorder. These in turn are controlled by factors such as inter-pigment geometries and their interactions with protein and membrane environments. | |
<ref> https://www.sciencedirect.com/science/article/pii/S002228360300024X?via%3Dihub </ref> | <ref> https://www.sciencedirect.com/science/article/pii/S002228360300024X?via%3Dihub </ref> | ||
| - | + | The light-absorbing pigments, bacteriochlorophyll a (Bchl a) and carotenoids, | |
| - | + | are non-covalently bound to two low-molecular-weight hydrophobic apoproteins, a and p. The native complexes are oligomers | |
| - | + | of these components. <ref>https://doi.org/10.1038/374517a0</ref> | |
| - | + | ||
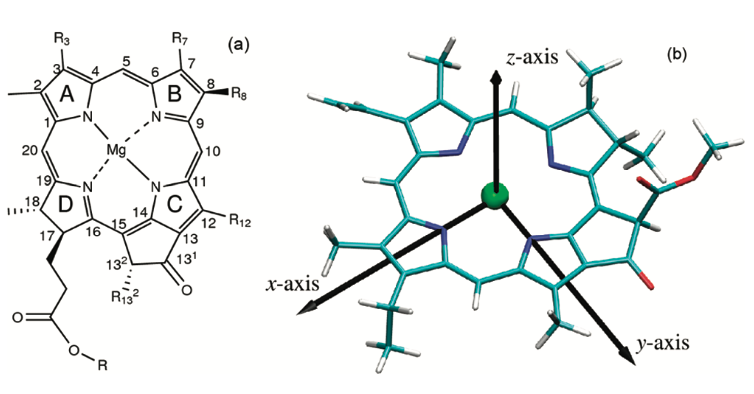
Each individual light-harvesting complex is composed of oligomers of short peptides (α and β) with associated pigments (Hawthornthwaite & Cogdell, 1991). αβ apoproteins with their non-covalently bound carotenoid and bacteriochlorophyll (Bchl ) pigments form the multi-subunit complexes LH1 and LH2 | Each individual light-harvesting complex is composed of oligomers of short peptides (α and β) with associated pigments (Hawthornthwaite & Cogdell, 1991). αβ apoproteins with their non-covalently bound carotenoid and bacteriochlorophyll (Bchl ) pigments form the multi-subunit complexes LH1 and LH2 | ||
| Line 27: | Line 35: | ||
The differences between the LH1 and LH2 complexes reside in their protein/pigment stoichiometry and modes of oligomerization. Structural studies have shown that LH2 complexes are formed from eight or nine αβ subunit oligomers | The differences between the LH1 and LH2 complexes reside in their protein/pigment stoichiometry and modes of oligomerization. Structural studies have shown that LH2 complexes are formed from eight or nine αβ subunit oligomers | ||
| - | Structural studies have shown that LH2 complexes are formed from nine ab <scene name='91/911263/Alpha_carbon_lhc_ba_ii/1'>Alpha Beta subunits</scene>, organised in a ring of inner a and outer b-peptides. In between the b-peptides and close to the cytoplasmic surface, aswell as Near the | + | Structural studies have shown that LH2 complexes are formed from nine ab <scene name='91/911263/Alpha_carbon_lhc_ba_ii/1'>Alpha Beta subunits</scene>, organised in a ring of inner a (hollow cylinder of radius 18 A) and outer b-peptides (outer cylinder of radius 34 A). The a-apoprotein contains 53 amino acids, and the p-apoprotein 41.<ref>https://doi.org/10.1038/374517a0</ref> |
| + | |||
| + | The Bchl a-binding histidines of the a (His 31) and | ||
| + | p (His 30) apoproteins face outwards and inwards, respectively, | ||
| + | forming a complete ring of 18 overlapping Bchl a molecules. For | ||
| + | these molecules, the planes of the bacteriochlorins are parallel to | ||
| + | the membrane normal and their centres are approximately loA | ||
| + | from the presumed periplasmic membrane surface. The nine | ||
| + | remaining Bchl a molecules are packed between the p-apoprotein | ||
| + | helices a further 16.5 A into the membrane with their bacteriochlorin rings parallel to the membrane surface. | ||
| + | |||
| + | . Circular | ||
| + | dichroism analysis of these absorption bands has led to the conclusion that the Bchl a molecules that absorb at 800 nm are | ||
| + | largely monomeric, whereas those absorbing at 850 nm are | ||
| + | strongly exciton-coupled5 | ||
| + | • Conserved histidine residues in the | ||
| + | apoproteins have been shown, by resonance Raman spectroscopy, to be liganded to the Mg at the centre of the Bchl a that | ||
| + | absorbs at 850 nm<ref>https://doi.org/10.1038/374517a0</ref> | ||
| + | |||
| + | |||
| + | |||
| + | In between the b-peptides and close to the cytoplasmic surface, aswell as Near the | ||
periplasmic surface, and between a and b- peptides, are <scene name='91/911263/Bacteriochlorophylls/1'>Bacteriochlorophylls</scene>, they are responsible for near infrared absorption, being called B800 and B850. | periplasmic surface, and between a and b- peptides, are <scene name='91/911263/Bacteriochlorophylls/1'>Bacteriochlorophylls</scene>, they are responsible for near infrared absorption, being called B800 and B850. | ||
<scene name='91/911263/Rhodopin_glucoside/1'> Carotenoids</scene>pigments are also present and absorb in the visible part of the spectrum and perform the additional role of protection against photo-induced oxidation, in the case of the LHC II the carotenoid present is rhodopin glucoside. | <scene name='91/911263/Rhodopin_glucoside/1'> Carotenoids</scene>pigments are also present and absorb in the visible part of the spectrum and perform the additional role of protection against photo-induced oxidation, in the case of the LHC II the carotenoid present is rhodopin glucoside. | ||
| Line 34: | Line 63: | ||
==About the pigments== | ==About the pigments== | ||
| + | Most photosynthetics pigments are chlorophylls (Chl), bacteriochlorophylls (BChl), and carotenoids, they represent the keystone for energy storage in photosynthetic organisms | ||
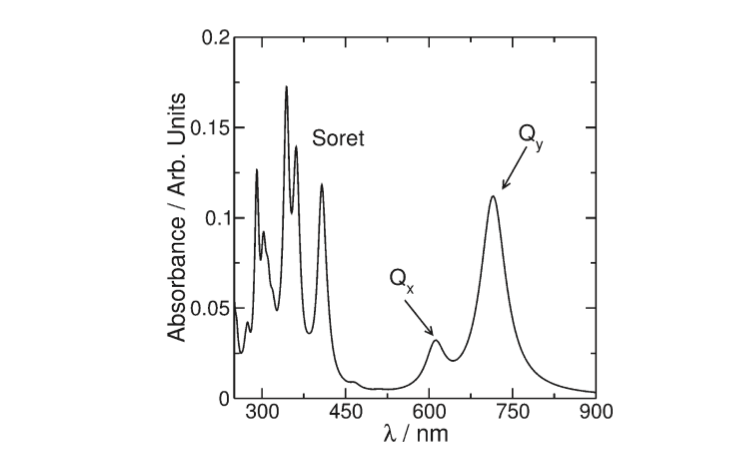
The BChl a Qy transition dipole moments are responsible for the near infrared absorption by Bchl a molecules at generic wavelengths 800 nm and 850 nm, and for this reason these pigments are called B800 and B850.<ref> https://doi.org/10.1016/S0022-2836(03)00024-X</ref> | The BChl a Qy transition dipole moments are responsible for the near infrared absorption by Bchl a molecules at generic wavelengths 800 nm and 850 nm, and for this reason these pigments are called B800 and B850.<ref> https://doi.org/10.1016/S0022-2836(03)00024-X</ref> | ||
Revision as of 22:24, 5 June 2022
Light Harvesting Complex II
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ https://doi.org/10.1021/jp203826q
- ↑ https://doi.org/10.1038/374517a0
- ↑ https://www.sciencedirect.com/science/article/pii/S002228360300024X?via%3Dihub
- ↑ https://doi.org/10.1038/374517a0
- ↑ https://doi.org/10.1038/374517a0
- ↑ https://doi.org/10.1038/374517a0
- ↑ https://doi.org/10.1016/S0022-2836(03)00024-X
- ↑ https://bmc1.utm.utoronto.ca/~vijay/prototype_V12/physChem/molExcit/p08/index.html
- ↑ https://doi.org/10.1021/jp203826q
- ↑ https://doi.org/10.1021/jp203826q
- ↑ https://doi.org/10.1016/S0022-2836(03)00024-X
- ↑ https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Valence_Bond_Theory/Delocalization_of_Electrons
- ↑ https://doi.org/10.1038/374517a0