Trypsin
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
== Function == | == Function == | ||
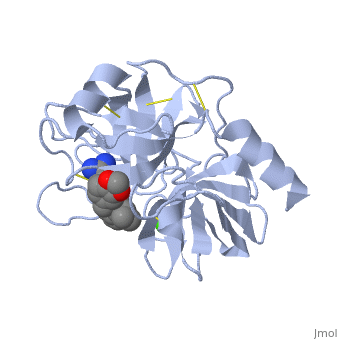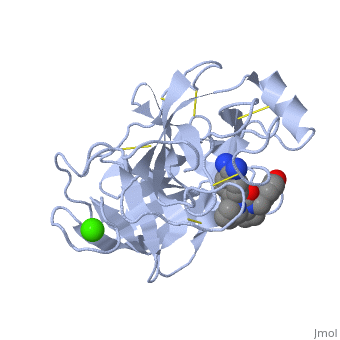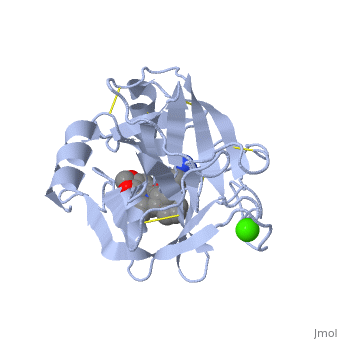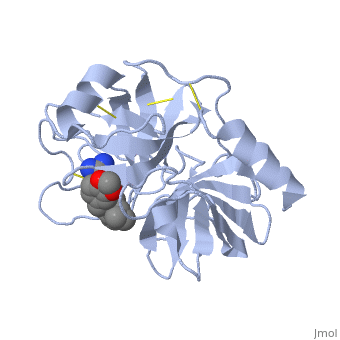
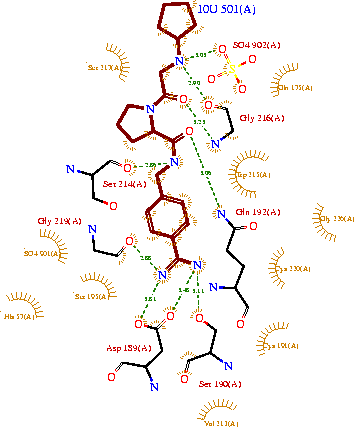
| - | '''Trypsin''' is a medium size globular protein that functions as a pancreatic serine protease. This enzyme hydrolyzes bonds by cleaving peptides on the C-terminal side of the amino acid residues lysine and arginine. It has also been shown that cleavage will not occur if there is a proline residue on the carboxyl side of the cleavage site. Trypsin was first discovered in 1876 by Kuhne, who investigated the proteolytic activity of the enzyme. In 1931 the enzyme was purified by crystallization by Norothrop and Kunitz and later in 1974 the three dimensional structure of trypsin was determined. Throughout the 1990's the role of trypsin in hereditary pancreatitis and the mutation that causes it was discovered. Today trypsin is used in the development of cell and tissue protocols, as well as in the medical field to determine the role of trypsin in pancreatic diseases<ref>Trypsin. 2010. 30 October 2010 <http://www.worthington-biochem.com/tyr/default.html></ref>. | + | '''Trypsin''' or '''serine protease 1''' is a medium size globular protein that functions as a pancreatic serine protease. This enzyme hydrolyzes bonds by cleaving peptides on the C-terminal side of the amino acid residues lysine and arginine. It has also been shown that cleavage will not occur if there is a proline residue on the carboxyl side of the cleavage site. Trypsin was first discovered in 1876 by Kuhne, who investigated the proteolytic activity of the enzyme. In 1931 the enzyme was purified by crystallization by Norothrop and Kunitz and later in 1974 the three dimensional structure of trypsin was determined. Throughout the 1990's the role of trypsin in hereditary pancreatitis and the mutation that causes it was discovered. Today trypsin is used in the development of cell and tissue protocols, as well as in the medical field to determine the role of trypsin in pancreatic diseases<ref>Trypsin. 2010. 30 October 2010 <http://www.worthington-biochem.com/tyr/default.html></ref>. |
[[Image:Tryogen.gif |thumb|left|Trypsinogen]] | [[Image:Tryogen.gif |thumb|left|Trypsinogen]] | ||
{{Clear}} | {{Clear}} | ||
Current revision
| |||||||||||
References
- ↑ Trypsin. 2010. 30 October 2010 <http://www.worthington-biochem.com/tyr/default.html>
- ↑ Trypsin. 30 October 2010 <http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/analytical-enzyme/trypsin.html>.
- ↑ Image From: http://chemistry.umeche.maine.edu/MAT500/Peptidase1.html
- ↑ Trypsin. 2010. 30 October 2010 <http://www.worthington-biochem.com/tyr/default.html>
- ↑ Pratt, C.W., Voet, D., Voet, J.G. Fundamentals of Biochemistry - Life at the Molecular Level - Third Edition. Voet, Voet and Pratt, 2008.
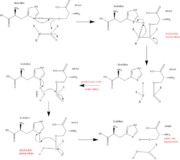
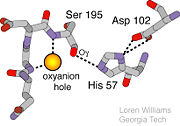
- ↑ Structural Biochemistry. 10 June 2010. 30 October 2010.<http://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme/Catalytic_Triad>.
- ↑ Image From:

- ↑ Williams, Loren. Georgia Tech. http://www2.chemistry.gatech.edu/~1W26/bcourse_information/6521/protein/serine_protease/triad_1/html.
- ↑ Structural Biochemistry. 10 June 2010. 30 October 2010.<http://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme/Catalytic_Triad>.
- ↑ Pratt, C.W., Voet, D., Voet, J.G. Fundamentals of Biochemistry - Life at the Molecular Level - Third Edition. Voet, Voet and Pratt, 2008.
- ↑ Joshi RS, Mishra M, Tamhane VA, Ghosh A, Sonavane U, Suresh CG, Joshi R, Gupta VS, Giri AP. The remarkable efficiency of a Pin-II proteinase inhibitor sans two conserved disulfide bonds is due to enhanced flexibility and hydrogen bond density in the reactive site loop. J Biomol Struct Dyn. 2012 Dec 20. PMID:23256852 doi:10.1080/07391102.2012.745378
- ↑ 12.0 12.1 Green TR, Ryan CA. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776-7. PMID:17836138 doi:10.1126/science.175.4023.776
- ↑ Kong L, Ranganathan S. Tandem duplication, circular permutation, molecular adaptation: how Solanaceae resist pests via inhibitors. BMC Bioinformatics. 2008;9 Suppl 1:S22. PMID:18315854 doi:10.1186/1471-2105-9-S1-S22
- ↑ Johnson R, Narvaez J, An G, Ryan C. Expression of proteinase inhibitors I and II in transgenic tobacco plants: effects on natural defense against Manduca sexta larvae. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9871-5. PMID:2602379
- ↑ Duan X, Li X, Xue Q, Abo-el-Saad M, Xu D, Wu R. Transgenic rice plants harboring an introduced potato proteinase inhibitor II gene are insect resistant. Nat Biotechnol. 1996 Apr;14(4):494-8. PMID:9630927 doi:10.1038/nbt0496-494
- ↑ Nielsen KJ, Heath RL, Anderson MA, Craik DJ. Structures of a series of 6-kDa trypsin inhibitors isolated from the stigma of Nicotiana alata. Biochemistry. 1995 Nov 7;34(44):14304-11. PMID:7578034
- ↑ Scanlon MJ, Lee MC, Anderson MA, Craik DJ. Structure of a putative ancestral protein encoded by a single sequence repeat from a multidomain proteinase inhibitor gene from Nicotiana alata. Structure. 1999 Jul 15;7(7):793-802. PMID:10425681
- ↑ Lee MC, Scanlon MJ, Craik DJ, Anderson MA. A novel two-chain proteinase inhibitor generated by circularization of a multidomain precursor protein. Nat Struct Biol. 1999 Jun;6(6):526-30. PMID:10360353 doi:10.1038/9293
- ↑ Schirra HJ, Scanlon MJ, Lee MC, Anderson MA, Craik DJ. The solution structure of C1-T1, a two-domain proteinase inhibitor derived from a circular precursor protein from Nicotiana alata. J Mol Biol. 2001 Feb 9;306(1):69-79. PMID:11178894 doi:10.1006/jmbi.2000.4318
- ↑ Schirra HJ, Craik DJ. Structure and folding of potato type II proteinase inhibitors: circular permutation and intramolecular domain swapping. Protein Pept Lett. 2005 Jul;12(5):421-31. PMID:16029154
- ↑ Schirra HJ, Anderson MA, Craik DJ. Structural refinement of insecticidal plant proteinase inhibitors from Nicotiana alata. Protein Pept Lett. 2008;15(9):903-9. PMID:18991765
- ↑ Schirra HJ, Guarino RF, Anderson MA, Craik DJ. Selective removal of individual disulfide bonds within a potato type II serine proteinase inhibitor from Nicotiana alata reveals differential stabilization of the reactive-site loop. J Mol Biol. 2010 Jan 22;395(3):609-26. Epub 2009 Nov 17. PMID:19925809 doi:10.1016/j.jmb.2009.11.031
- ↑ Li XQ, Zhang T, Donnelly D. Selective loss of cysteine residues and disulphide bonds in a potato proteinase inhibitor II family. PLoS One. 2011 Apr 11;6(4):e18615. PMID:21494600 doi:10.1371/journal.pone.0018615
- ↑ Barrette-Ng IH, Ng KK, Cherney MM, Pearce G, Ryan CA, James MN. Structural basis of inhibition revealed by a 1:2 complex of the two-headed tomato inhibitor-II and subtilisin Carlsberg. J Biol Chem. 2003 Jun 27;278(26):24062-71. Epub 2003 Apr 8. PMID:12684499 doi:10.1074/jbc.M302020200
- ↑ Dunse KM, Kaas Q, Guarino RF, Barton PA, Craik DJ, Anderson MA. Molecular basis for the resistance of an insect chymotrypsin to a potato type II proteinase inhibitor. Proc Natl Acad Sci U S A. 2010 Aug 24;107(34):15016-21. Epub 2010 Aug 9. PMID:20696921 doi:10.1073/pnas.1009327107
- ↑ Tamhane VA, Giri AP, Kumar P, Gupta VS. Spatial and temporal expression patterns of diverse Pin-II proteinase inhibitor genes in Capsicum annuum Linn. Gene. 2009 Aug 1;442(1-2):88-98. Epub 2009 Apr 22. PMID:19393726 doi:10.1016/j.gene.2009.04.012
- ↑ Tamhane VA, Chougule NP, Giri AP, Dixit AR, Sainani MN, Gupta VS. In vivo and in vitro effect of Capsicum annum proteinase inhibitors on Helicoverpa armigera gut proteinases. Biochim Biophys Acta. 2005 Mar 11;1722(2):156-67. Epub 2005 Jan 12. PMID:15715970 doi:10.1016/j.bbagen.2004.12.017
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Eran Hodis, Leah Bowlin, David Canner, Glenn Jones, Ben Hallowell, Karl Oberholser, Jaime Prilusky