User:Clara Costa D'Elia/Sandbox 1
From Proteopedia
| Line 40: | Line 40: | ||
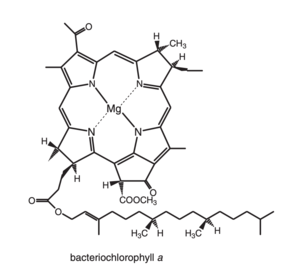
<scene name='91/911263/Bcl_with_mg/1'>Bacteriochlorophyll a</scene>It is the principal chlorophyll-type pigment in the majority of anoxygenic photosynthetic bacteria and acomodated several structural changes in comparinson to the typical clorophin pigments, specially regarded its symmetry, witch impose effects on its spectral properties. | <scene name='91/911263/Bcl_with_mg/1'>Bacteriochlorophyll a</scene>It is the principal chlorophyll-type pigment in the majority of anoxygenic photosynthetic bacteria and acomodated several structural changes in comparinson to the typical clorophin pigments, specially regarded its symmetry, witch impose effects on its spectral properties. | ||
| - | [[Image:Bacteriochlorophylla.png]] <ref>Blankenship, R. E. Molecular Mechanisms of Photosynthesis; | + | [[Image:Bacteriochlorophylla.png|300px|left|thumb|Bacteriochlorophylla]] |
| + | |||
| + | <ref>Blankenship, R. E. Molecular Mechanisms of Photosynthesis; | ||
Blackwell Science Ltd.: Oxford, U.K., 2002.</ref> | Blackwell Science Ltd.: Oxford, U.K., 2002.</ref> | ||
| Line 54: | Line 56: | ||
Acordig to <ref>Blankenship, R. E. Molecular Mechanisms of Photosynthesis; | Acordig to <ref>Blankenship, R. E. Molecular Mechanisms of Photosynthesis; | ||
Blackwell Science Ltd.: Oxford, U.K., 2002.</ref>, The spectra of photosynthethic pigments in gereral can be described by using the “four orbital” model. In this model there are for p molecular orbitals involved, simplified as HOMOs (highest occupied molecular orbitals) and LUMOs ( lowest unoccupied molecular orbitals). | Blackwell Science Ltd.: Oxford, U.K., 2002.</ref>, The spectra of photosynthethic pigments in gereral can be described by using the “four orbital” model. In this model there are for p molecular orbitals involved, simplified as HOMOs (highest occupied molecular orbitals) and LUMOs ( lowest unoccupied molecular orbitals). | ||
| - | [[Image:Orbitalss.png]] | + | [[Image:Orbitalss.png|300px|right|thumb|Orbitalss]] |
The two lowest-energy transitions are called the Q bands, they are in the visible region | The two lowest-energy transitions are called the Q bands, they are in the visible region | ||
of the spectrum and are the most important for the photophysics, and the two higher-energy ones are known as the B bands or Soret bands, and are found in the UV region composing of a large series of electronic transitions.<ref> https://doi.org/10.1021/jp203826q </ref> | of the spectrum and are the most important for the photophysics, and the two higher-energy ones are known as the B bands or Soret bands, and are found in the UV region composing of a large series of electronic transitions.<ref> https://doi.org/10.1021/jp203826q </ref> | ||
<ref>https://doi.org/10.1038/374517a0</ref>. | <ref>https://doi.org/10.1038/374517a0</ref>. | ||
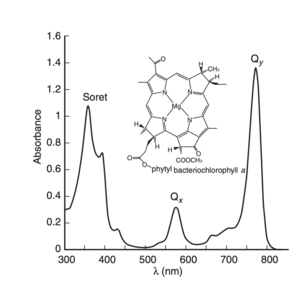
| - | [[Image:Bacteriochlorophyllaabs.png]] | + | [[Image:Bacteriochlorophyllaabs.png|300px|left|thumb|subtitle]] |
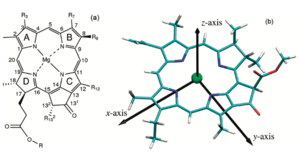
In order to further understand how the pigments respond to light excitation, its important to visually the moleculas x and y axis. By convention, the y molecular axis is defined as the axis passing through the N atoms of rings A and C as show in the figure; | In order to further understand how the pigments respond to light excitation, its important to visually the moleculas x and y axis. By convention, the y molecular axis is defined as the axis passing through the N atoms of rings A and C as show in the figure; | ||
| - | [[Image:Qy 2.png]]<ref> https://doi.org/10.1021/jp203826q </ref>. Acording to <ref>Blankenship, R. E. Molecular Mechanisms of Photosynthesis; | + | [[Image:Qy 2.png|300px|right|thumb|subtitle]]<ref> https://doi.org/10.1021/jp203826q </ref>. Acording to <ref>Blankenship, R. E. Molecular Mechanisms of Photosynthesis; |
Blackwell Science Ltd.: Oxford, U.K., 2002.</ref> "The longest-wavelength transition is invariably polarized along the y | Blackwell Science Ltd.: Oxford, U.K., 2002.</ref> "The longest-wavelength transition is invariably polarized along the y | ||
axis of the molecule and is therefore known as the Qy transition. This means that the absorption will be strongest if the electric vector of plane-polarized exciting light is parallel to the y molecular axis of the pigment. The exciting light couples to the p electrons of the | axis of the molecule and is therefore known as the Qy transition. This means that the absorption will be strongest if the electric vector of plane-polarized exciting light is parallel to the y molecular axis of the pigment. The exciting light couples to the p electrons of the | ||
| Line 73: | Line 75: | ||
==Energy transfer mechanism== | ==Energy transfer mechanism== | ||
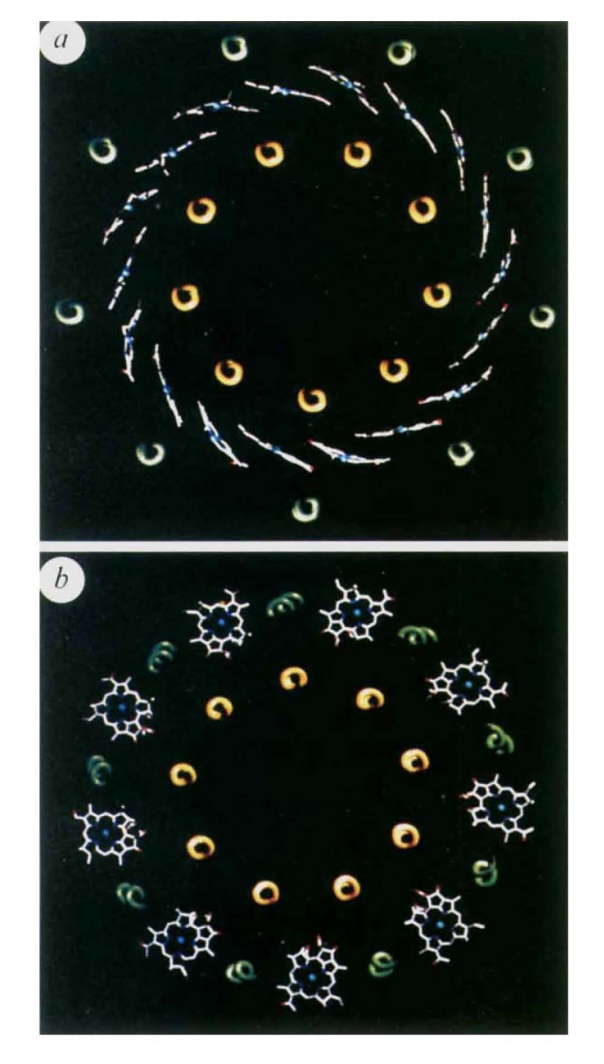
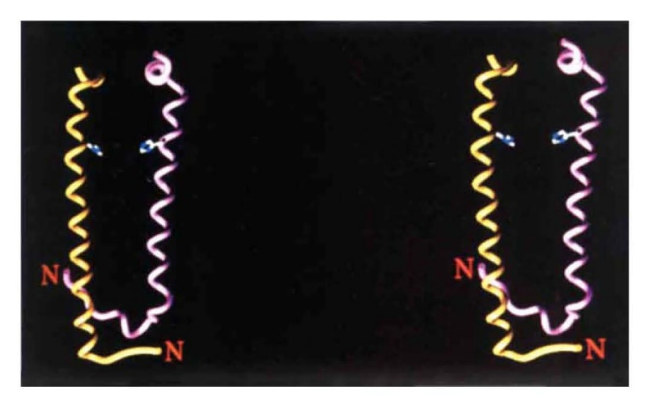
| - | When a Bchl molecule is excited by light, its first excited singlet | + | When a Bchl molecule is excited by light, its first excited singlet state lasts for a few nanoseconds!. The light-harvesting system must be able to transfer the absorbed energy to the reaction centre in a shorter time than this. Some of the important features that allow this to take place are revealed by the structure reported here. Previous biophysical studies (reviewed in ref. 1) have shown that energy transfer within the LH2 complex can occur from the BSOO to the BS50 BChl a molecules in 0.7 ps. Once the energy reaches the BS50 molecules, it is rapidly transferred among them. This is seen as an ultrafast depolarization of the excited state on the 200-300 fs timescale. Energy transfer from LH2 to LHI occurs in the 5-20 ps time range, but with LH2 alone the decay of the S50 nm excited singlet state takes 1.1 ns. The ring of BS50 Bchl a molecules acts rather like a 'storage ring', with the excited state rapidly delocalized over a large area. The delocalization is facilitated by a highly hydrophobic environment which reduces the dielectric constant, allowing coupling over large distances. The energy is then available for transfer from any part of the ring to any neighbouring LHI complex. It is clear from electron microscopy imaging of the LHI complex 13 , and from a comparison of the primary structures3 of the LH2 and LH I complexes, that the structure of the LHI complex is similar to that of LH2 (with a larger ring). With such a ring structure, there is no requirement for the |
| - | state lasts for a few nanoseconds!. The light-harvesting system | + | LHI complex to have a special orientation to receive energy from the LH2 ring. Furthermore, because the BS75 Bchl a |
| - | must be able to transfer the absorbed energy to the reaction | + | molecules in LHI are liganded to homologous histidine residues, as in LH2, it is likely that the BS50 and BS75 bacteriochlorophyll rings will be at the same point in the membrane. The overall effect will be to allow energy transfer from any LH2 to any LHI complex that is within range, without regard to the orientation of either complex. This reduction in the dimensionality of the process will lead to a further kinetic gain. Previous studies have shown that the carotenoid in this LH2 complex acts as an efficient accessory light-harvesting pigment (>50%)7. The excited singlet lifetime of carotenoids is usually less than 10 pS8. Therefore, if energy transfer is to compete successfully with these rapid de-excitation processes, the carotenoid must be located very close to the acceptor bacteriochlorophylls8, as seen in the structure. |
| - | centre in a shorter time than this. Some of the important features | + | |
| - | that allow this to take place are revealed by the structure | + | |
| - | reported here. | + | |
| - | Previous biophysical studies (reviewed in ref. 1) have shown | + | |
| - | that energy transfer within the LH2 complex can occur from the | + | |
| - | BSOO to the BS50 BChl a molecules in 0.7 ps. Once the energy | + | |
| - | reaches the BS50 molecules, it is rapidly transferred among them. | + | |
| - | This is seen as an ultrafast depolarization of the excited state on | + | |
| - | the 200-300 fs timescale. Energy transfer from LH2 to LHI | + | |
| - | occurs in the 5-20 ps time range, but with LH2 alone the decay | + | |
| - | of the S50 nm excited singlet state takes 1.1 ns. | + | |
| - | The ring of BS50 Bchl a molecules acts rather like a 'storage | + | |
| - | ring', with the excited state rapidly delocalized over a large area. | + | |
| - | The delocalization is facilitated by a highly hydrophobic | + | |
| - | environment which reduces the dielectric constant, allowing | + | |
| - | coupling over large distances. The energy is then available for | + | |
| - | transfer from any part of the ring to any neighbouring LHI | + | |
| - | complex. It is clear from electron microscopy imaging of the | + | |
| - | LHI complex 13 , and from a comparison of the primary | + | |
| - | structures3 of the LH2 and LH I complexes, that the structure | + | |
| - | of the LHI complex is similar to that of LH2 (with a larger | + | |
| - | ring). With such a ring structure, there is no requirement for the | + | |
| - | LHI complex to have a special orientation to receive energy | + | |
| - | from the LH2 ring. Furthermore, because the BS75 Bchl a | + | |
| - | molecules in LHI are liganded to homologous histidine residues, | + | |
| - | as in LH2, it is likely that the BS50 and BS75 bacteriochlorophyll | + | |
| - | rings will be at the same point in the membrane. The overall | + | |
| - | effect will be to allow energy transfer from any LH2 to any LHI | + | |
| - | complex that is within range, without regard to the orientation | + | |
| - | of either complex. This reduction in the dimensionality of the | + | |
| - | process will lead to a further kinetic gain. | + | |
| - | Previous studies have shown that the carotenoid in this LH2 | + | |
| - | complex acts as an efficient accessory light-harvesting pigment | + | |
| - | (>50%)7. The excited singlet lifetime of carotenoids is usually | + | |
| - | less than 10 pS8. Therefore, if energy transfer is to compete successfully with these rapid de-excitation processes, the carotenoid | + | |
| - | must be located very close to the acceptor bacteriochlorophylls8, | + | |
| - | as seen in the structure. | + | |
<ref>https://doi.org/10.1038/374517a0</ref> | <ref>https://doi.org/10.1038/374517a0</ref> | ||
| - | == Structural highlights == | ||
| - | ######################################## | ||
| - | The chlorophylls all contain two major absorption bands, one in the blue or near UV region and one in the red or near IR region.The lack of a significant absorption in the green | ||
| - | region gives the chlorophylls their characteristic green or blue–green color. These absorption bands are pÆp* transitions,involving the electrons in the conjugated psystem | ||
| - | of the chlorin macrocycle. | ||
| - | |||
| - | |||
| - | Each individual light-harvesting complex is composed of oligomers of short peptides (α and β) with associated pigments (Hawthornthwaite & Cogdell, 1991). αβ apoproteins with their non-covalently bound carotenoid and bacteriochlorophyll (Bchl ) pigments form the multi-subunit complexes LH1 and LH2 | ||
| Line 129: | Line 86: | ||
and the B850 pigments. | and the B850 pigments. | ||
| - | |||
| - | |||
| - | </StructureSection> | ||
==Structure== | ==Structure== | ||
| - | <StructureSection load='1KZU' size='350' side='right' caption='test (PDB entry [[1KZU]])' scene=''> | ||
The differences between the LH1 and LH2 complexes reside in their protein/pigment stoichiometry and modes of oligomerization. Structural studies have shown that LH2 complexes are formed from eight or nine αβ subunit oligomers | The differences between the LH1 and LH2 complexes reside in their protein/pigment stoichiometry and modes of oligomerization. Structural studies have shown that LH2 complexes are formed from eight or nine αβ subunit oligomers | ||
Revision as of 16:58, 14 June 2022
Contents |
Your Heading Here (maybe something like 'Structure')
</StructureSection>==Light Harvesting Complex II==
|
This is a default text for your page Clara Costa D'Elia/Sandbox 1. Click above on edit this page to modify. Be careful with the < and > signs. You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
introduction
Photosynthesis is the main source of energy used by life on earth nowadays. "The beginning of photosynthesis starts with the absorption of sunlight by an arrangement of photosynthetic pigments embedded into a proteic matrix called the light harvesting (LH) antenna complexes. The excitation energy of photosynthetic pigments is then transferred to the photosynthetic reaction center where it is converted into chemical energy". .[3]
Photosynthesis is divided in a few steps in a way that the antenna system does not do any chemistry, since it only works by transfering energy in the state of excited electrons between molecules. According to [4] this is only possible due to a weak energetic coupling of the antenna pigments, which are bound to proteins in highly specific associations.
Is it important to notice that this page describes the LHC II of purple bacterias, more specifically the one found in Rhodopseudomonas acidophila, which caries a similiar name but has nothing to do to the LHC II of plants and algae.
IN general Purple bacteria contain two types of antenna complexes, integral membrane proteins, the light harvesting complex I and II. LHI associates with the reaction center and is always present, constituting part of the "core complex" as explained by [5]. Light harvest complex to, also called LHII is located on the periphery of this core complex and its not always present, since its produced by the bacteria as an acessory complex depending on the avaiability of light leves encountered by the organism, in order to increase its range of absorption(Zuber & Brunisholz, 1991). Its important to notice that Both types of complex are built on a similar modular principle.
When purple bacteria are grown under anaerobic conditions they incorporate the photosynthetic apparatus described above into invaginated intracytoplasmic phospholipid membranes.[6]
Function
Your Heading Here (Light capture)
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ https://doi.org/10.1021/jp203826q
- ↑ Blankenship, R. E. Molecular Mechanisms of Photosynthesis; Blackwell Science Ltd.: Oxford, U.K., 2002.
- ↑ https://doi.org/10.1038/374517a0
- ↑ https://doi.org/10.1038/374517a0
- ↑ https://www.sciencedirect.com/science/article/pii/S002228360300024X?via%3Dihub
- ↑ https://doi.org/10.1038/374517a0
- ↑ Blankenship, R. E. Molecular Mechanisms of Photosynthesis; Blackwell Science Ltd.: Oxford, U.K., 2002.
- ↑ https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Valence_Bond_Theory/Delocalization_of_Electrons
- ↑ https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Valence_Bond_Theory/Delocalization_of_Electrons
- ↑ Blankenship, R. E. Molecular Mechanisms of Photosynthesis; Blackwell Science Ltd.: Oxford, U.K., 2002.
- ↑ https://doi.org/10.1021/jp203826q
- ↑ https://doi.org/10.1038/374517a0
- ↑ https://doi.org/10.1021/jp203826q
- ↑ Blankenship, R. E. Molecular Mechanisms of Photosynthesis; Blackwell Science Ltd.: Oxford, U.K., 2002.
- ↑ https://bmc1.utm.utoronto.ca/~vijay/prototype_V12/physChem/molExcit/p08/index.html
- ↑ https://doi.org/10.1038/374517a0
- ↑ https://doi.org/10.1038/374517a0
- ↑ https://doi.org/10.1038/374517a0