User:Sabrina K.K. Komatsu/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 29: | Line 29: | ||
The tower domain (TD) is also involved with DNA binding. G-segment binds with a beta-sheet region, while another region composed by a + b sheets mediates domain-domain interactions (Chang et al, 2013).The TD DNA-binding surface juxtaposes with the DNA-intercalating wing-2 motif of WHD, extending the DNA-binding surface of the WHD and anchoring the outer end of the bent G-segment DNA. Interestingly, the structural conservation observed for the fixed packing between the tower domain and WHD suggests their assembly as an integral unit for the production and stabilization of DNA-bending (Chang et al, 2013). | The tower domain (TD) is also involved with DNA binding. G-segment binds with a beta-sheet region, while another region composed by a + b sheets mediates domain-domain interactions (Chang et al, 2013).The TD DNA-binding surface juxtaposes with the DNA-intercalating wing-2 motif of WHD, extending the DNA-binding surface of the WHD and anchoring the outer end of the bent G-segment DNA. Interestingly, the structural conservation observed for the fixed packing between the tower domain and WHD suggests their assembly as an integral unit for the production and stabilization of DNA-bending (Chang et al, 2013). | ||
| + | ==Operation== | ||
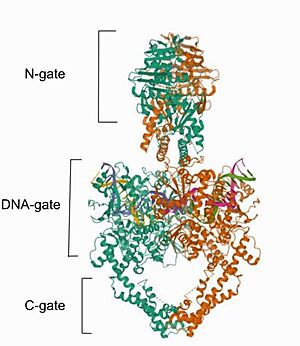
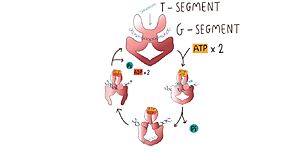
| + | Type II topoisomerases operation involves a complex mechanism of controlled association and dissociation of subunit dimerization elements. Top2A starts out with its N gates dissociated allowing it to bind a DNA duplex passing it through the N gate and binding at the DNA gate (G-segment). Binding at the DNA gate causes a significant one ~150 degree bend, promoted by an isoleucine present in the WHD domain. The N gate contains an ATPase region and ATP binding here triggers the binding of a second DNA duplex at the N gate (T-segment).[[Image:Top9-7010-.jpg|300px|right|thumb|Image adapted from Chang, C.-C., Wang, Y.-R., Chen, S.-F., Wu, C.-C., & Chan, N.-L. (2013). New insights into DNA-binding by type IIA topoisomerases. Current Opinion in Structural Biology, 23(1), 125–133. doi:10.1016/j.sbi.2012.11.011]] Binding of ATP also causes the previously separated N gates to dimerize and close locking in the T-segment. Closing the N gates emulates conformation change that promote the cleavage of the G segment, which is catalyzed by a pair of symmetrically-related tyrosines 9,10, in conjunction with a Mg2+ ion-binding in the TOPRIM fold. Besides that, the 150 degree bend on the duplex puts bonds under significant stress, facilitating the cleavage. Once the G segment has been cleaved, it remains bound at the DNA gate, but the DNA gate opens to allow the T segment to exit through the open C gate. Than, the DNA gate closes again to religate the G segment before releasing it. Strand passage and gate openings are coordinated by defined ATPase domains12–14 which use ATP binding and hydrolysis to promote conformation changes. | ||
| + | In more simple terms, in the type IIA reaction cycle, one double-stranded DNA is bound and cleaved by the enzyme, while a second duplex is transported through the break (Wendorff et al, 2012). | ||
| - | |||
| - | Operation: | ||
| - | |||
| - | Figura adaptada de Chang, C.-C., Wang, Y.-R., Chen, S.-F., Wu, C.-C., & Chan, N.-L. (2013). New insights into DNA-binding by type IIA topoisomerases. Current Opinion in Structural Biology, 23(1), 125–133. doi:10.1016/j.sbi.2012.11.011 | ||
| - | |||
| - | Type II topoisomerases operation involves a complex mechanism of controlled association and dissociation of subunit dimerization elements. Top2A starts out with its N gates dissociated allowing it to bind a DNA duplex passing it through the N gate and binding at the DNA gate (G-segment). Binding at the DNA gate causes a significant one ~150 degree bend, promoted by an isoleucine present in the WHD domain. The N gate contains an ATPase region and ATP binding here triggers the binding of a second DNA duplex at the N gate (T-segment). Binding of ATP also causes the previously separated N gates to dimerize and close locking in the T-segment. Closing the N gates emulates conformation change that promote the cleavage of the G segment, which is catalyzed by a pair of symmetrically-related tyrosines 9,10, in conjunction with a Mg2+ ion-binding in the TOPRIM fold. Besides that, the 150 degree bend on the duplex puts bonds under significant stress, facilitating the cleavage. Once the G segment has been cleaved, it remains bound at the DNA gate, but the DNA gate opens to allow the T segment to exit through the open C gate. Than, the DNA gate closes again to religate the G segment before releasing it. Strand passage and gate openings are coordinated by defined ATPase domains12–14 which use ATP binding and hydrolysis to promote conformation changes. | ||
| - | In more simple terms, in the type IIA reaction cycle, one double-stranded DNA is bound and cleaved by the enzyme, while a second duplex is transported through the break (Wendorff et al, 2012). | ||
| - | // | ||
== Relevance == | == Relevance == | ||
As mentioned before, TOP2 mechanism of DNA cleavage is not simple, and errors can result in cell death. This aspect of TOP2 has been explored by anticancer studies, which showed that TOP2 inhibitors increase the steady-state level of cleavage complexes, promoting the formation of cytotoxic DNA lesions in cancer cells (Wu et al, 2011). . TOP2-targeting agents are divided into two categories: "Topo poisons" and "Topo inhibitors", based on their mechanisms of action. TOP2 poisons do not prevent TOP2-DNA interaction, but convert the essential enzyme into a highly cytotoxic DNA-damaging agent, due to a stabilized linked DNA strand-passing intermediate, allowing the generation of double strand breaks, but not religation of the strands. "TOP2 inhibitors" interrupt the binding of TOP2 and DNA by binding enzymes to inhibit TOP2 catalytic activity but do not generate increases in the levels of TOP2 covalent complexes (Chen, Qiu & Shen, 2012). | As mentioned before, TOP2 mechanism of DNA cleavage is not simple, and errors can result in cell death. This aspect of TOP2 has been explored by anticancer studies, which showed that TOP2 inhibitors increase the steady-state level of cleavage complexes, promoting the formation of cytotoxic DNA lesions in cancer cells (Wu et al, 2011). . TOP2-targeting agents are divided into two categories: "Topo poisons" and "Topo inhibitors", based on their mechanisms of action. TOP2 poisons do not prevent TOP2-DNA interaction, but convert the essential enzyme into a highly cytotoxic DNA-damaging agent, due to a stabilized linked DNA strand-passing intermediate, allowing the generation of double strand breaks, but not religation of the strands. "TOP2 inhibitors" interrupt the binding of TOP2 and DNA by binding enzymes to inhibit TOP2 catalytic activity but do not generate increases in the levels of TOP2 covalent complexes (Chen, Qiu & Shen, 2012). | ||
It has been shown that TOP2α is a better target than TOP2β, once targeting the last one was responsible for the development of secondary malignancy. Besides, TOP2α is found more in rapidly growing cancer cells, once its essential for cell growth. So, in conclusion, specific inhibition of TOP2α resulted in significant antitumor action, suggesting that TOP2α-targeted therapy is a valuable approach for cancer chemotherapy and shows the importance of studying the enzyme itself (Chen, Qiu & Shen, 2012). | It has been shown that TOP2α is a better target than TOP2β, once targeting the last one was responsible for the development of secondary malignancy. Besides, TOP2α is found more in rapidly growing cancer cells, once its essential for cell growth. So, in conclusion, specific inhibition of TOP2α resulted in significant antitumor action, suggesting that TOP2α-targeted therapy is a valuable approach for cancer chemotherapy and shows the importance of studying the enzyme itself (Chen, Qiu & Shen, 2012). | ||
| - | |||
| - | |||
| - | == Operation == | ||
| - | |||
| - | Top2A starts out with its N gates dissociated allowing it to bind a DNA duplex passing it through the N gate and binding at the DNA gate (G-segment). Binding at the DNA gate causes a significant one ~150 degree bend, promoted by an isoleucine present in the WHD domain. The N gate contains an ATPase region and ATP binding here triggers the binding of a second DNA duplex at the N gate (T-segment). Binding of ATP also causes the previously separated N gates to dimerize and close locking in the T-segment. Closing the N gates emulates conformation change that promote the cleavage of the G segment, which is catalyzed by a pair of symmetrically-related tyrosines 9,10, in conjunction with a Mg2+ ion-binding in the TOPRIM fold. Besides that, the 150 degree bend on the duplex puts bonds under significant stress, facilitating the cleavage. Once the G segment has been cleaved, it remains bound at the DNA gate, but the DNA gate opens to allow the T segment to exit through the open C gate. Than, the DNA gate closes again to religate the G segment before releasing it. Strand passage and gate openings are coordinated by defined ATPase domains12–14 which use ATP binding and hydrolysis to promote conformation changes. | ||
| - | In more simple terms, in the type IIA reaction cycle, one double-stranded DNA is bound and cleaved by the enzyme, while a second duplex is transported through the break. | ||
| - | |||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 21:48, 18 June 2022
DNA Topoisomerase II
| |||||||||||