User:Sabrina K.K. Komatsu/Sandbox 1
From Proteopedia
(Difference between revisions)
m |
|||
| Line 2: | Line 2: | ||
<StructureSection load='4FM9' size='340' side='right' caption='DNA topoisomerase II alpha' scene=''> | <StructureSection load='4FM9' size='340' side='right' caption='DNA topoisomerase II alpha' scene=''> | ||
DNA Topoisomerases (TOP) are enzymes capable of solving topological problems in DNA molecules during replication, transcription, recombination and chromatin remodeling by introducing temporary breaks in the DNA. Besides that, these enzymes can modulate the level of DNA supercoiling to facilitate protein interaction with the molecule and to prevent supercoiling relationed stress <ref name="sorvete">PMID: 33480441</ref> . There are several types of topoisomerases that are different in structure and function, but basically, the topos are divided based on whether they catalyze single- (type I) or double-stranded (type II) DNA breaks <ref name="sorvete" />. | DNA Topoisomerases (TOP) are enzymes capable of solving topological problems in DNA molecules during replication, transcription, recombination and chromatin remodeling by introducing temporary breaks in the DNA. Besides that, these enzymes can modulate the level of DNA supercoiling to facilitate protein interaction with the molecule and to prevent supercoiling relationed stress <ref name="sorvete">PMID: 33480441</ref> . There are several types of topoisomerases that are different in structure and function, but basically, the topos are divided based on whether they catalyze single- (type I) or double-stranded (type II) DNA breaks <ref name="sorvete" />. | ||
| - | Within type two topoisomerases there are two classes: A and B. Class B is found only in archaea and type A is present in eukarya and bacteria. Humans have two types of 2A topoisomerases: isoforms alpha and beta. Topoisomerase 2 alpha has a larger role in chromosome segregation and DNA replication and tends to be expressed in more proliferating tissues, which makes topoisomerase 2 alpha a cancer cell marker and the target of anti-cancer drugs. | + | Within type two topoisomerases there are two classes: A and B. Class B is found only in archaea and type A is present in eukarya and bacteria. Humans have two types of 2A topoisomerases: isoforms alpha and beta. Topoisomerase 2 alpha has a larger role in chromosome segregation and DNA replication and tends to be expressed in more proliferating tissues, which makes topoisomerase 2 alpha a cancer cell marker and the target of anti-cancer drugs. TOP2A is responsible for the selective cleaving, rearranging and religation of DNA strands, which helps untangle the chromosome, thereby changing the state of DNA in the cell <ref name="sorvete" />. |
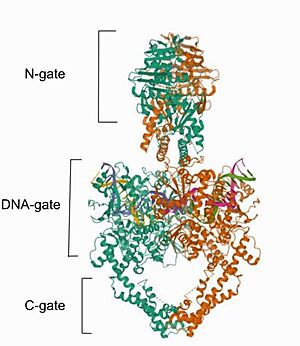
| - | Mammalian DNA topoisomerase IIα (human) IIA is a homodimeric protein, in which each subunit structure can be broken down into three major components that are connected by hinged like regions: the N gate, the DNA gate and the C gate <ref>DOI:10.1016/j.febslet.2011.08.051</ref>. | + | Mammalian DNA topoisomerase IIα (human) (IIA) is a homodimeric protein, in which each subunit structure can be broken down into three major components that are connected by hinged like regions: the N gate, the DNA gate and the C gate <ref>DOI:10.1016/j.febslet.2011.08.051</ref>. |
== Function == | == Function == | ||
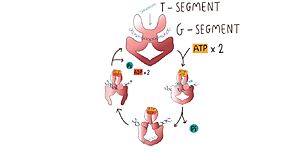
| - | During replication, transcription and cell division, DNA passes for various states of coiling, so sometimes DNA can become too coiled, which, without alleviation, can cause DNA brakes or arrest of the replication, cell division or transcription. To prevent breakages from happening, | + | During replication, transcription and cell division, DNA passes for various states of coiling, so sometimes DNA can become too coiled, which, without alleviation, can cause DNA brakes or arrest of the replication, cell division or transcription. To prevent breakages from happening, TOP2a cuts both strands of the DNA duplex, passes another DNA duplex through the cleaved one and relegate the cut duplex to release tension. This is a risky task, once not completed properly, it damages the state of the chromatin and can result in cell death <ref name="rasmol" /> <ref>DOI:10.1146/annurev.biochem.70.1.369</ref>. |
| - | In addition to the better-known function of undoing super-coilings in DNA, TOP2a was shown to be important for comosomal condensation and maintenance of chromosomal structure, since chromatic compaction appears to arise, in part, due to the interaction between | + | In addition to the better-known function of undoing super-coilings in DNA, TOP2a was shown to be important for comosomal condensation and maintenance of chromosomal structure, since chromatic compaction appears to arise, in part, due to the interaction between TOP2a and complexes of structural maintenance of chromosomes (SMC complexes).TOP2a is also involved in chromosome segregation during anaphase and in chromatid resolution at ribosomal DNA (rDNA) <ref name="rasmol" /> <ref>DOI:10.1073/pnas.2001760117</ref>. |
===Structure=== | ===Structure=== | ||
| - | Cryo-EM reconstructions of the | + | Cryo-EM reconstructions of the TOP2α nucleoprotein complex showed that Mammalian <scene name='91/914441/Topoisomerase_ii_alfa/1'>DNA topoisomerase IIα (human) IIA</scene> is a homodimeric protein, in whitch each subunit structure can be broken down into three major components that are connected by hinged like regions: the N gate, that contains the ATPase domain, the DNA gate and the C gate<ref name="chocolate">DOI:10.1038/s41467-021-23136-6</ref>. Biochemical and structural studies have shown that the ATPase domain or N-gate, and the DNA binding/cleavage domain forming the DNA- and C-gates, are allosterically connected, a key feature of its activity <ref name="chocolate"/>. Furthermore, the literature contains various descriptions of the structures of the N-gate and DNA-gate, but the C-gate is less explored. [[Image:Image- Top2.jpg|300px|right|thumb|Image from the RCSB PDB (https://www.rcsb.org/structure/6ZY8) of PDB ID 6ZY8, Cryo-EM structure of the entire Human topoisomerase II alpha in State 2.]] |
The protein DNA Topoisomerase IIα (human) has two <scene name='91/914441/Chains/2'>chains</scene> one represented in <scene name='91/914441/Green/1'>green</scene> and one in <scene name='91/914441/Gray/1'>gray</scene>, both bounded to <scene name='91/914441/Dna/1'>double-stranded DNA</scene>. | The protein DNA Topoisomerase IIα (human) has two <scene name='91/914441/Chains/2'>chains</scene> one represented in <scene name='91/914441/Green/1'>green</scene> and one in <scene name='91/914441/Gray/1'>gray</scene>, both bounded to <scene name='91/914441/Dna/1'>double-stranded DNA</scene>. | ||
| Line 23: | Line 23: | ||
===The DNA gate=== | ===The DNA gate=== | ||
| - | Within the DNA gate there are three important domains: the topoisomerase primases (toprim domain), the winged helix domain (WHD domain or 5Y-CAP)) and the tower domain. The TOPRIM domain contains a DxD motif, where metal ion binding occurs due to two aspartates at positions 541 and 543, which coordinates a single magnesium 2 plus ion quelation,and a glutamate residue, that donates a proton to the sugar hydroxyl of the DNA during cleavage and abstracting the proton from the 3ʹ-OH during re-ligation. The TOPRIM domain also contributes to DNA binding, due to conserved residues, namely the EGDS and PLRGK motifs, which interact with the G-segment. Furthermore, the TOPRIM employs a distinct insertion, called insertion 2, composed of a short helix followed by a long loop, known for accommodation of DNA <ref name="lara"/>. | + | Within the DNA gate there are three important domains: the topoisomerase primases (toprim domain), the winged helix domain (WHD domain or 5Y-CAP)) and the tower domain. |
| + | |||
| + | ===The TOPRIM domain=== | ||
| + | The TOPRIM domain contains a DxD motif, where metal ion binding occurs due to two aspartates at positions 541 and 543, which coordinates a single magnesium 2 plus ion quelation,and a glutamate residue, that donates a proton to the sugar hydroxyl of the DNA during cleavage and abstracting the proton from the 3ʹ-OH during re-ligation. The TOPRIM domain also contributes to DNA binding, due to conserved residues, namely the EGDS and PLRGK motifs, which interact with the G-segment. Furthermore, the TOPRIM employs a distinct insertion, called insertion 2, composed of a short helix followed by a long loop, known for accommodation of DNA <ref name="lara"/>. | ||
| + | |||
| + | ===THE Winged Helix domain=== | ||
The WHD contains a helix-turn-helix fold, common in DNA-binding proteins, that have catalytic tyrosine residues in the C-terminal helix (also termed the ‘recognition helix’), responsible for forming a reversible covalent bond with the 5ʹ-scissile DNA phosphate. Besides that, The WHD also holds an isoleucine, which intercalates into the G-segment (the first segment of DNA duplex that enter the enzyme) producing a ∼150° bend, promoting DNA cleavage. The cleaving of the DNA backbone occurs in a bipartite active site formed by the TOPRIM DxD motif and the active site tyrosine of the WHD, which physically associate in different orientations during the enzyme's cycle of action <ref name="lara"/>. | The WHD contains a helix-turn-helix fold, common in DNA-binding proteins, that have catalytic tyrosine residues in the C-terminal helix (also termed the ‘recognition helix’), responsible for forming a reversible covalent bond with the 5ʹ-scissile DNA phosphate. Besides that, The WHD also holds an isoleucine, which intercalates into the G-segment (the first segment of DNA duplex that enter the enzyme) producing a ∼150° bend, promoting DNA cleavage. The cleaving of the DNA backbone occurs in a bipartite active site formed by the TOPRIM DxD motif and the active site tyrosine of the WHD, which physically associate in different orientations during the enzyme's cycle of action <ref name="lara"/>. | ||
Revision as of 22:50, 18 June 2022
DNA Topoisomerase II
| |||||||||||