User:Luize Nóbrega e Silva/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
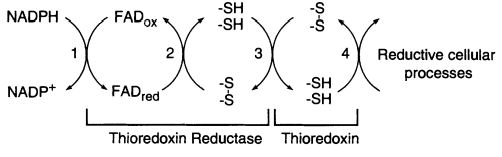
The <scene name='91/914431/1cl0_color/2'>thioredoxin reductase</scene> of E. coli is a member of the flavoenzymes, responsible for catalyzing the reduction of the redox protein thioredoxin, by using NADPH. <ref>PMID:7557016</ref> The <scene name='91/914431/1f6m/3'>Trx System</scene> has its role as a protein disulfide reductase comprising thioredoxin reductase (TrxR), thioredoxin (Trx) and NADPH. Thioredoxin is the main disulfide reductase responsible for keeping intracellular proteins reduced. The redox reactions are catalyzed by thioredoxin reductase (TrxR) by taking electrons from NADPH and transferring them to the FAD domain of the oxidized Trx (Trs-S2). Electrons are then transferred to the redox active disulfide in the N-terminal of one subunit of TrxR, finally to the C-terminal active site of the other subunit, generating a a dithiol in reduced thioredoxin, Trx-(SH)2. <ref>PMID:20494123</ref> <ref>PMID:11012661</ref> | The <scene name='91/914431/1cl0_color/2'>thioredoxin reductase</scene> of E. coli is a member of the flavoenzymes, responsible for catalyzing the reduction of the redox protein thioredoxin, by using NADPH. <ref>PMID:7557016</ref> The <scene name='91/914431/1f6m/3'>Trx System</scene> has its role as a protein disulfide reductase comprising thioredoxin reductase (TrxR), thioredoxin (Trx) and NADPH. Thioredoxin is the main disulfide reductase responsible for keeping intracellular proteins reduced. The redox reactions are catalyzed by thioredoxin reductase (TrxR) by taking electrons from NADPH and transferring them to the FAD domain of the oxidized Trx (Trs-S2). Electrons are then transferred to the redox active disulfide in the N-terminal of one subunit of TrxR, finally to the C-terminal active site of the other subunit, generating a a dithiol in reduced thioredoxin, Trx-(SH)2. <ref>PMID:20494123</ref> <ref>PMID:11012661</ref> | ||
The activity of thioredoxin reductase on thioredoxin is importante in E. coli for more than a reason. Despite this enzyme has a conserved role as a high-capacity hydrogen donor systems for reductive enzymes, it also has evolved to specialized functions, such as participating in filamentous E. coli phage assembly and its T7 DNA replication. Furthermore, thioredoxin has demonstrated an important role in defense against oxidative stress or in control of apoptosis. Most of these functions are dependent of the disulfide reductase activity, which is only possible with the NADPH and thioredoxin reductase supply. <ref>PMID:11012661</ref> | The activity of thioredoxin reductase on thioredoxin is importante in E. coli for more than a reason. Despite this enzyme has a conserved role as a high-capacity hydrogen donor systems for reductive enzymes, it also has evolved to specialized functions, such as participating in filamentous E. coli phage assembly and its T7 DNA replication. Furthermore, thioredoxin has demonstrated an important role in defense against oxidative stress or in control of apoptosis. Most of these functions are dependent of the disulfide reductase activity, which is only possible with the NADPH and thioredoxin reductase supply. <ref>PMID:11012661</ref> | ||
| - | + | [[Image:Trxr reaction.JPG]] | |
== Structure == | == Structure == | ||
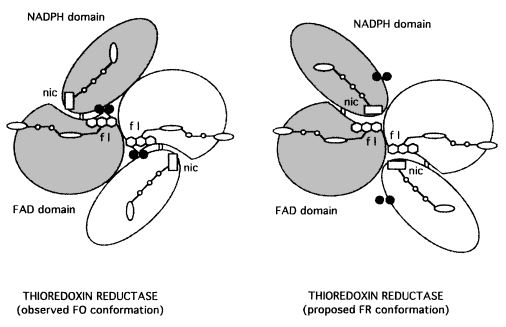
The enzyme thioredoxin reductase from Escherichia coli is a homodimer, in which each monomer contains one FAD and one redox active disulfide. The first part of the FAD binding domain (FAD-i), the NADPH binding domain, and the last part of the FAD binding domain (FAD-2) is formed by the polypeptide chain of each monomer. The connection between FAD and NADPH domains is a two-stranded, antiparallel B-sheet, and each one has a secondary and tertiary structure. <ref>PMID:7557016</ref> | The enzyme thioredoxin reductase from Escherichia coli is a homodimer, in which each monomer contains one FAD and one redox active disulfide. The first part of the FAD binding domain (FAD-i), the NADPH binding domain, and the last part of the FAD binding domain (FAD-2) is formed by the polypeptide chain of each monomer. The connection between FAD and NADPH domains is a two-stranded, antiparallel B-sheet, and each one has a secondary and tertiary structure. <ref>PMID:7557016</ref> | ||
Revision as of 18:44, 19 June 2022
Introduction: Thioredoxin system
| |||||||||||