Lipoprotein Lipase (LPL) complexed with GPIHBP1
From Proteopedia
(Difference between revisions)
(New page: ==Your Heading Here (maybe something like 'Structure')== <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> This is a default text for you...) |
|||
| Line 1: | Line 1: | ||
| - | = | + | =Lipoprotein Lipase (LPL) complexed with GPIHBP1= |
| - | <StructureSection load=' | + | <StructureSection load='6e7k' size='350' frame='true' side='right' caption='Lipoprotein Lipase - 6E7K' scene='87/877603/Lplwcolors/10'> |
| - | + | ||
| - | + | ||
| - | == | + | ==Introduction== |
| + | <div style="text-align: left;"> | ||
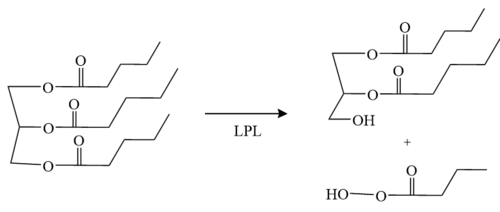
| + | LPL, [http://en.wikipedia.org/wiki/Lipoprotein_lipase lipoprotein lipase] is an enzyme that affects the breakdown of [http://en.wikipedia.org/wiki/Triglyceride triglycerides] (Figure 1) which are carried from various organs to the blood by molecules called [http://en.wikipedia.org/wiki/Lipoprotein lipoproteins]. LPL is found on the surface of cells lining the [http://en.wikipedia.org/wiki/Capillary capillaries] within muscles and fatty tissue. | ||
| + | [[Image:lplsimplemech.png|500 px|center]] | ||
| - | + | '''Figure 1:''' Conversion of triglyceride to diglyceride by LPL. | |
| - | = | + | LPL was identified more than 60 years ago and studied by biochemists and physiologists intensely since. It wasn’t until recently that LPL’s detailed structure was determined due to LPL’s hydrolase domain susceptibility to unfolding. LMF1 and GPIHBP1, '''glycosylphosphatidylinositol-anchored high-density lipoprotein–binding protein 1''' , were discovered to be required for proper folding and enzymatic activity of LPL. LMF1,''' lipase maturation factor 1''', is a [http://en.wikipedia.org/wiki/Chaperone_(protein) chaperone protein] that is responsible for proper folding and secretion of LPL. Through the use of X-ray crystallography, it was also discovered that LPL is a [http://en.wikipedia.org/wiki/Monomer monomer] rather than the previously believed [http://en.wikipedia.org/wiki/Protein_dimer homodimer] <ref name="Birrane">PMID:30559189</ref>. |
| + | </div> | ||
| - | == | + | ===Function=== |
| + | LPL is located within the interstitial space independently and remains stranded there if not acted upon by GPIHBP1<ref name="Davies">PMID:20620994</ref>. GPIHBP1 then captures LPL in the interstitial spaces and shuttles it across [http://en.wikipedia.org/wiki/Endothelium endothelial cells] into the capillary lumen. Once in the capillaries, the LPL-GPIHBP1 complex catalyzes the breakdown of triglycerides in the blood. In doing so, it prevents high levels of triglycerides in the plasma to provide nutrients for vital tissues <ref name="Birrane">PMID:30559189</ref>. | ||
| + | ===Significance=== | ||
| + | Lipoprotein lipase deficiency leads to [http://.en.wikipedia.org/wiki/Hypertriglyceridemia hypertriglyceridemia] (elevated levels of triglycerides in the blood) also known as [http://en.wikipedia.org/wiki/Lipoprotein_lipase_deficiency chylomicronemia]. This can go on to increase [http://en.wikipedia.org/wiki/Insulin_resistance insulin resistance] and the risk of [http://en.wikipedia.org/wiki/Obesity obesity]. | ||
| - | + | == Structure == | |
| + | ===Overall Structure=== | ||
| + | Overall, LPL is a monomer but in the <scene name='87/877603/Chain_a_and_chain_b/6'>experimental crystal structure</scene> it is paired with another monomer. The two monomers in the experimental structure are identical besides orientation. The two monomers are oriented head to tail. Biologically, LPL is identified as a heterodimer. LPL is also complexed with GPIHBP1 (shown as cyan) and is essential for LPL to remain stable and avoid [http://en.wikipedia.org/wiki/Denaturation_(biochemistry) denaturation]. | ||
| + | ===LPL=== | ||
| + | LPL has two main domains, the larger N-terminus domain containing the active site and the smaller C-terminus domain. These two domains are connected via a peptide linker hinge. LPL also contains a large basic patch and a single calcium ion. Additionally, LPL consists of two N-linked [http://en.wikipedia.org/wiki/Glycan glycans] (N70, N386) which likely contribute to the correct folding of LPL due to the attached [http://en.wikipedia.org/wiki/Oligosaccharide oligosaccharides]. Five [http://en.wikipedia.org/wiki/Disulfide disulfide bonds] contribute to the stabilization throughout LPL’s structure. Lastly, the active site in the larger N-terminus domain is lined with hydrophobic residues <ref name="Birrane">PMID:30559189</ref>. | ||
| + | ====N-terminus of LPL==== | ||
| + | The N-terminus of LPL is made up of 6 [http://en.wikipedia.org/wiki/Alpha_helix α-helices] and 10 [http://en.wikipedia.org/wiki/Beta_sheet β-sheets] known as the <scene name='87/877603/A-b_domain/3'>N-terminal α/β-hydrolase domain</scene>. Additionally, α/β hydrolase harbors the catalytic triad<ref name="Wong">PMID:8144612</ref>. | ||
| + | ====C-terminus of LPL==== | ||
| + | The C-terminus of LPL consists of 12 β-sheets and is known as the <scene name='87/877603/B-domain/3'>C-terminal flattened β-barrel domain</scene>. The β-sheets are interacting giving a shape that resembles an elongated cylinder or barrel<ref name="Wong">PMID:8144612</ref>. | ||
| + | ===GPIHBP1=== | ||
| + | GPIHBP1 is a <scene name='87/877603/3-fingered/3'>three-fingered</scene> LU (Ly6/uPAR) domain. GPIHBP1 is stabilized by five disulfide bonds and is also known for its N-terminal intrinsically disordered acidic domain<ref name="Birrane">PMID:30559189</ref>. The N-terminal intrinsically disordered acidic domain (residues 21-61) has not been resolved experimentally and is not shown in the crystal structure. | ||
| + | ===LPL-GPIHBP1 complex=== | ||
| + | ====Interaction of LPL and GPIHBP1==== | ||
| + | GPIHBP1’s LU domain interacts with LPL’s C-terminal domain via <scene name='87/877636/Hydrophobic_interact_lpl_gpi/1'>hydrophobic interactions</scene>. This is largely due to the [http://en.wikipedia.org/wiki/Hydrophobic_effect hydrophobic effect] and stabilization. The acidic N-terminal domain of GPIHBP1 (residues 21–61) is disordered and not visible in the structure, which is presumably due to dynamic interaction with the large basic patch on the LPL<ref name="Birrane">PMID:30559189</ref>. | ||
| + | ====Calcium Ion Coordination==== | ||
| + | The <scene name='87/877636/Calcium_ion_coordination/2'>calcium ion</scene> has been shown to convert inactive LPL to the active dimer form. The calcium ion is coordinated by residues A194, R197, S199, D201, and D202. Mutations in the coordinating residues can give rise to detrimental metabolic diseases<ref name="Birrane">PMID:30559189</ref>. The crystal structures of LPL revealed that the carboxylic acid side chain of D201 significantly aids in the coordination of LPL with the calcium ion. If D201 is mutated to a valine, for example, LPL can no longer fold correctly, and thus, LPL secretion from cells is inhibited due to the loss of the carboxylic acid side chain <ref name="Birrane">PMID:30559189</ref>. | ||
| + | ===Active Site=== | ||
| + | The <scene name='87/878236/Active_site_4_20/4'>active site</scene> of LPL is composed of multiple pieces. The <scene name='87/878236/Active_site_4_20/5'>hydrophobic entry</scene> to the binding site outlines the general structure and provides considerable stability to the active site. The <scene name='87/878236/Active_site_lid_region_fixed/6'>lid region</scene> is also an important component of the active site, occupying residues 243-266, which is vital for the recognition of substrates. The <scene name='87/878236/Active_site_w_catalytic_residu/7'>catalytic residues</scene> consist of the [http://en.wikipedia.org/wiki/Catalytic_triad#:~:text=A%20catalytic%20triad%20is%20a,lipases%20and%20%CE%B2%2Dlactamases) catalytic triad] (residues H268, S159 and D183) and the [http://en.wikipedia.org/wiki/Oxyanion_hole#:~:text=An%20oxyanion%20hole%20is%20a,amides%20or%20positively%20charged%20residues oxyanion hole], consisting of residues <scene name='87/877636/Activesite_oxyanionhole/4'>L160 and W82</scene>. The catalytic residues catalyze the reaction of the typical substrate of LPL, triglycerides, and the oxyanion hole is responsible for aiding in the stability of the transition state of substrates. The main chain nitrogens stabilize the tetrahedral intermediate <ref name="Birrane">PMID:30559189</ref>. | ||
| + | ====Mechanism==== | ||
| + | [[Image:finalmechlpl.png|650 px|center]] | ||
| + | |||
| + | '''Figure 2:''' The hydrolase mechanism catalyzed by LPL. | ||
| + | |||
| + | a. The triglyceride binds to LPL’s lipid-binding region in an open lid conformation. | ||
| + | |||
| + | b. The oxygen on S159 is made more [http://en.wikipedia.org/wiki/Nucleophile nucleophilic]. This happens via histidine hydrogen bonding with the hydrogen on S159’s alcohol group. | ||
| + | |||
| + | c. The nucleophilic oxygen attacks the carbonyl carbon of one of the fatty acid chains. | ||
| + | |||
| + | d. This pushes electrons up onto the carbonyl oxygen, creating a [http://www.chem.ucla.edu/~harding/IGOC/T/tetrahedral_intermediate.html tetrahedral intermediate]. This is the oxyanion hole which is stabilized by main chain nitrogen atoms of W82 and L160. | ||
| + | |||
| + | e.One of the lone pairs on the oxygen (in the oxyanion hole) creates a double bond carbon. | ||
| + | |||
| + | f. The oxygen-carbon bond between the single fatty acid chain and the [http://en.wikipedia.org/wiki/Diglyceride diglyceride] is cleaved. | ||
| + | |||
| + | g. H268 hydrogen bonds water, making the oxygen a better nucleophile. Water attacks the carbonyl carbon. | ||
| + | |||
| + | h. The carboxylic acid is formed and the S159 bond is cleaved and re-protonated via H268. | ||
| + | |||
| + | i. The active site is now back in its original state. | ||
| + | |||
| + | ===Inhibitor=== | ||
| + | The <scene name='87/878236/Active_site_w_inhibitor_bound/11'>inhibitor</scene>, M3D, was a vital piece in unraveling the correct structure of LPL. With the inhibitor bound, LPL’s active site and lid region become visible and crystallizable, thus this inhibitor is what allowed the first complete crystal structure of LPL to come into existence <ref name="Birrane">PMID:30559189</ref>. This inhibitor also revealed the correct orientation of H268, a residue involved in the catalytic triad. Originally, the hydrogen bonding did not align with substrates in the original crystal structure; however, when this inhibitor was bound, the H268 was discovered to be flipped, thus aligning the hydrogen bonds in the correct orientation <ref name="Arora">PMID:31072929</ref>. | ||
| + | == Disease == | ||
| + | ===Mutations=== | ||
| + | ====D201V==== | ||
| + | <scene name='87/877636/D201_mutation/12'>D201V</scene> is a mutation that is found to cause [http://en.wikipedia.org/wiki/Lipoprotein_lipase_deficiency chylomicronemia]. Chylomicronemia is when the body cannot break down lipids properly<ref name="Beigneux">PMID:17403372</ref>. The side chain of aspartate 201 is one of the coordination sites for the calcium ion of LPL. The mutation to hydrophobic valine means the loss of this coordination site<ref name="Birrane">PMID:30559189</ref>. This mutation adversely affects the folding of LPL and thus affects the secretion of LPL, overall decreasing the activity of LPL<ref name="Birrane">PMID:30559189</ref>. This leads to high levels of triglycerides in the body <ref name="Falko">PMID:30183397</ref>. | ||
| + | |||
| + | ====M404R==== | ||
| + | <scene name='87/877636/M404r_1/4'>M404R</scene> is a mutation found within LPL that caused chylomicronemia in patients <ref name="Falko">PMID:30183397</ref>. The hydrophobic methionine is mutated to the larger and charged side chain of arginine. Originally it was thought to impact LPL secretion from cells. It was found that the M404R does not affect LPL secretion <ref name="Birrane">PMID:30559189</ref>. M404R interacts with the hydrophobic pocket of GPIHBP1’s finger 3 of its 3 fingered domain (V121, E122, T124, V126). The large, charged arginine repelled the hydrophobic pocket and does not fit well. This prevents proper binding and formation of the LPL-GPIHBP1 complex <ref name="Birrane">PMID:30559189</ref>. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | |||
| + | ==Student/Contributors== | ||
| + | *Ashrey Burley | ||
| + | *Allison Welz | ||
| + | *Hannah Wright | ||
Revision as of 20:05, 10 July 2022
Lipoprotein Lipase (LPL) complexed with GPIHBP1
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Birrane G, Beigneux AP, Dwyer B, Strack-Logue B, Kristensen KK, Francone OL, Fong LG, Mertens HDT, Pan CQ, Ploug M, Young SG, Meiyappan M. Structure of the lipoprotein lipase-GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc Natl Acad Sci U S A. 2018 Dec 17. pii: 1817984116. doi:, 10.1073/pnas.1817984116. PMID:30559189 doi:http://dx.doi.org/10.1073/pnas.1817984116
- ↑ Davies BS, Beigneux AP, Barnes RH 2nd, Tu Y, Gin P, Weinstein MM, Nobumori C, Nyren R, Goldberg I, Olivecrona G, Bensadoun A, Young SG, Fong LG. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 2010 Jul 7;12(1):42-52. doi: 10.1016/j.cmet.2010.04.016. PMID:20620994 doi:http://dx.doi.org/10.1016/j.cmet.2010.04.016
- ↑ 3.0 3.1 Wong H, Davis RC, Thuren T, Goers JW, Nikazy J, Waite M, Schotz MC. Lipoprotein lipase domain function. J Biol Chem. 1994 Apr 8;269(14):10319-23. PMID:8144612
- ↑ Arora R, Nimonkar AV, Baird D, Wang C, Chiu CH, Horton PA, Hanrahan S, Cubbon R, Weldon S, Tschantz WR, Mueller S, Brunner R, Lehr P, Meier P, Ottl J, Voznesensky A, Pandey P, Smith TM, Stojanovic A, Flyer A, Benson TE, Romanowski MJ, Trauger JW. Structure of lipoprotein lipase in complex with GPIHBP1. Proc Natl Acad Sci U S A. 2019 May 21;116(21):10360-10365. doi:, 10.1073/pnas.1820171116. Epub 2019 May 9. PMID:31072929 doi:http://dx.doi.org/10.1073/pnas.1820171116
- ↑ Beigneux AP, Davies BS, Gin P, Weinstein MM, Farber E, Qiao X, Peale F, Bunting S, Walzem RL, Wong JS, Blaner WS, Ding ZM, Melford K, Wongsiriroj N, Shu X, de Sauvage F, Ryan RO, Fong LG, Bensadoun A, Young SG. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007 Apr;5(4):279-91. doi: 10.1016/j.cmet.2007.02.002. PMID:17403372 doi:http://dx.doi.org/10.1016/j.cmet.2007.02.002
- ↑ 6.0 6.1 Falko JM. Familial Chylomicronemia Syndrome: A Clinical Guide For Endocrinologists. Endocr Pract. 2018 Aug;24(8):756-763. doi: 10.4158/EP-2018-0157. PMID:30183397 doi:http://dx.doi.org/10.4158/EP-2018-0157
Student/Contributors
- Ashrey Burley
- Allison Welz
- Hannah Wright