User:Arthur Migliatti/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
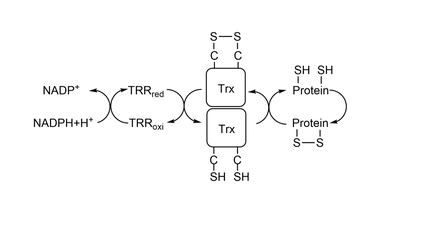
[[Image:ThioredoxinSystem.png|thumb|center|540x240px|Figure 1: Thioredoxin System.]] | [[Image:ThioredoxinSystem.png|thumb|center|540x240px|Figure 1: Thioredoxin System.]] | ||
| - | To reduce other proteins, first happens an attack from Cys32, creating an intermolecular | + | To reduce other proteins, first happens an attack from Cys32, creating an intermolecular disulfide bond, represented <scene name='91/911850/C32_s-s_c206/1'>here</scene> between residue Cys32 from Trx1 and residue Cys206 from '''[[MsrA]]'''.After it, residue Cys35 attacks Cys32, creating a <scene name='91/911850/Trx_cys_-_oxidized_-_diss_bond/4'>disulfide bond</scene> between the two cysteines in Trx1's catalytic site. To study the mechanism of reduction by Trx1 it is commom to use a wild-type Trx1 Cys35Ser. Since there is no other Cys to make a disulfide bond with Cys32, Trx1 is mainteined bonded to the other protein being studied by a intermolecular disulfide bond. |
== Structure == | == Structure == | ||
| - | Trx1 is a monomeric protein and weights around 12kDa. It is formed by one five-stranded beta sheet involved by 4 alpha helix, shown <scene name='91/911850/Secondary_structure/1'>here</scene>. The active site is located on a lump between betra strand 2, where Cys 35 is located, and alpha helix 2, where Cys32 is located. By being in the end of an alpha helix, <scene name='91/911850/Secondary_structure_cys32/1'>Cys32</scene> has a lower pKa, making it possible to reduce | + | Trx1 is a monomeric protein and weights around 12kDa. It is formed by one five-stranded beta sheet involved by 4 alpha helix, shown <scene name='91/911850/Secondary_structure/1'>here</scene>. The active site is located on a lump between betra strand 2, where Cys 35 is located, and alpha helix 2, where Cys32 is located. By being in the end of an alpha helix, <scene name='91/911850/Secondary_structure_cys32/1'>Cys32</scene> has a lower pKa, making it possible to reduce disulfide bonds.<ref>Holmgren, A. Thioredoxin Structure and Mechanism: Conformational Changes on Oxidation of the Active-Site Sulfhydryls to a Disulfide. Structure 1995, 3 (3), 239–243. https://doi.org/10.1016/S0969-2126(01)00153-8. |
</ref> | </ref> | ||
| Line 22: | Line 22: | ||
</ref>. | </ref>. | ||
| - | Since 1964, functions of Trx1 different than participating in cell division were discovered, as denitrosation and transnitrosation for example. Denitrosation is the removal of NO of a protein, and Trx1 does it by being temporarily S-nitrosataded on Cys32. Afterwards, Cys35 attacks Cys32 and forms a | + | Since 1964, functions of Trx1 different than participating in cell division were discovered, as denitrosation and transnitrosation for example. Denitrosation is the removal of NO of a protein, and Trx1 does it by being temporarily S-nitrosataded on Cys32. Afterwards, Cys35 attacks Cys32 and forms a disulfide bond, releasing HNO/NO to the medium. On the other hand, transnitrosation is the the nitrosation of other proteins, that the Trx1 of some species can do. |
| - | Although Trx1 from a great amount of organisms has only the catalytic site cysteines, the human form of Trx1 also has other <scene name='91/911850/Structural_cysteines/3'>3 structural cysteines</scene>, Cys 62, Cys 69 and Cys 73, which can act as regulators of the protein (<scene name='91/911850/Trx_yeast_-_62-69-73/2'>Cys62, Cys 69 and Cys73 of Trx1 from Saccharomices cerevisiae</scene>). <scene name='91/911850/Snocys69/2'>S-nitrosation of Trx on Cys69</scene> enhances its antiapoptotic function in some cases, although its not necessary for it.<ref>Tao, L.; Gao, E.; Bryan, N. S.; Qu, Y.; Liu, H.-R.; Hu, A.; Christopher, T. A.; Lopez, B. L.; Yodoi, J.; Koch, W. J.; Feelisch, M.; Ma, X. L. Cardioprotective Effects of Thioredoxin in Myocardial Ischemia and the Reperfusion Role of S-Nitrosation. Proc Natl Acad Sci U S A 2004, 101 (31), 11471–11476. https://doi.org/10.1073/pnas.0402941101.</ref>. Cys 73 has more than one function. Firstly, it is through this residue that Trx1 transnitrosate other proteins, the Trx of not all organisms are capable of doing transnitrosation. Another function is to make Trx1 a sensor of the redox state of the cell. When the cell is in a strong oxidizing state, Trx1 forms an homodimer connected by a <scene name='91/911850/Dimer/1'> | + | Although Trx1 from a great amount of organisms has only the catalytic site cysteines, the human form of Trx1 also has other <scene name='91/911850/Structural_cysteines/3'>3 structural cysteines</scene>, Cys 62, Cys 69 and Cys 73, which can act as regulators of the protein (<scene name='91/911850/Trx_yeast_-_62-69-73/2'>Cys62, Cys 69 and Cys73 of Trx1 from Saccharomices cerevisiae</scene>). <scene name='91/911850/Snocys69/2'>S-nitrosation of Trx on Cys69</scene> enhances its antiapoptotic function in some cases, although its not necessary for it.<ref>Tao, L.; Gao, E.; Bryan, N. S.; Qu, Y.; Liu, H.-R.; Hu, A.; Christopher, T. A.; Lopez, B. L.; Yodoi, J.; Koch, W. J.; Feelisch, M.; Ma, X. L. Cardioprotective Effects of Thioredoxin in Myocardial Ischemia and the Reperfusion Role of S-Nitrosation. Proc Natl Acad Sci U S A 2004, 101 (31), 11471–11476. https://doi.org/10.1073/pnas.0402941101.</ref>. Cys 73 has more than one function. Firstly, it is through this residue that Trx1 transnitrosate other proteins, the Trx of not all organisms are capable of doing transnitrosation. Another function is to make Trx1 a sensor of the redox state of the cell. When the cell is in a strong oxidizing state, Trx1 forms an homodimer connected by a <scene name='91/911850/Dimer/1'>disulfide bond between the Cys73 residue of each monomer</scene>. Since Cys73 is spacially close to the active site, the formation of a dimer prevents Trx1 from interacting with other proteins and reducing them (<font color='black'><b>black</b></font> = residues Cys73, <font color='magenta'><b>pink</b></font> = active site from monomer A, <font color='orange'><b>orange</b></font> = active site from monomer B). |
Revision as of 19:29, 26 July 2022
Introduction
| |||||||||||