Sandbox Reserved 1750
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
<div style="background-color:#fffaf0;"> | <div style="background-color:#fffaf0;"> | ||
== Publication Abstract from PubMed == | == Publication Abstract from PubMed == | ||
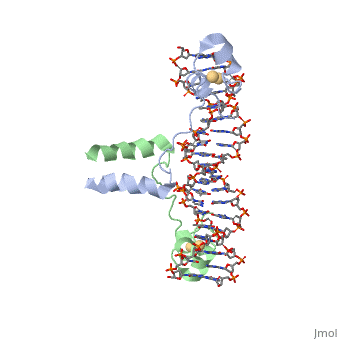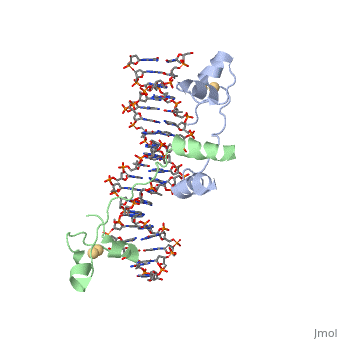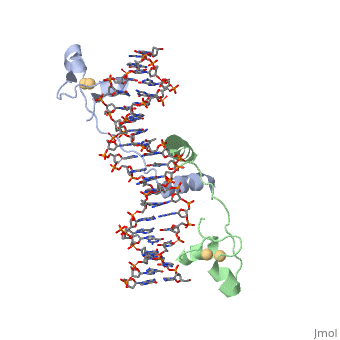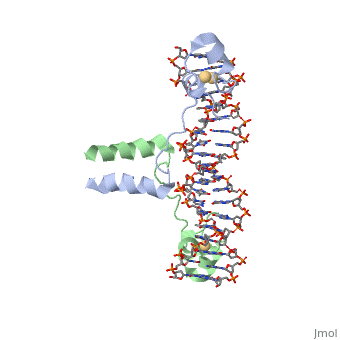
| - | A specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a <scene name='92/925538/Dimer/2'>dimer</scene> to a symmetrical 17-base-pair sequence. There is a compact <scene name='92/925538/Metal_binding_domain/1'>metal binding domain</scene> (residues 8-40), an <scene name='92/925538/Extended_linker/2'>extended linker</scene> (41-49), and an <scene name='92/925538/Alpha-helical_dimerization/1'>alpha-helical dimerization element</scene> (50-64). A small, Zn(2+)-containing domain recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. The binding domain contains <scene name='92/925538/Cysteine_metal_binding/1'>cysteine residues</scene> that coordinate to the metal as shown as cadmium. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4. | + | A specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a <scene name='92/925538/Dimer/2'>dimer</scene> to a symmetrical 17-base-pair sequence. There is a compact <scene name='92/925538/Metal_binding_domain/1'>metal binding domain</scene> (residues 8-40), an <scene name='92/925538/Extended_linker/2'>extended linker</scene> (41-49), and an <scene name='92/925538/Alpha-helical_dimerization/1'>alpha-helical dimerization element</scene> (50-64). A small, Zn(2+)-containing domain recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. The metal binding domain contains <scene name='92/925538/Cysteine_metal_binding/1'>cysteine residues</scene> that coordinate to the metal as shown as cadmium. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4. |
DNA recognition by GAL4: structure of a protein-DNA complex.,Marmorstein R, Carey M, Ptashne M, Harrison SC Nature. 1992 Apr 2;356(6368):408-14. PMID:1557122<ref>PMID:1557122</ref> | DNA recognition by GAL4: structure of a protein-DNA complex.,Marmorstein R, Carey M, Ptashne M, Harrison SC Nature. 1992 Apr 2;356(6368):408-14. PMID:1557122<ref>PMID:1557122</ref> | ||
Revision as of 20:56, 19 September 2022
DNA RECOGNITION BY GAL4: STRUCTURE OF A PROTEIN/DNA COMPLEX
| |||||||||||