1gux
From Proteopedia
OCA (Talk | contribs)
(New page: 200px<br /> <applet load="1gux" size="450" color="white" frame="true" align="right" spinBox="true" caption="1gux, resolution 1.85Å" /> '''RB POCKET BOUND TO ...)
Next diff →
Revision as of 15:02, 12 November 2007
|
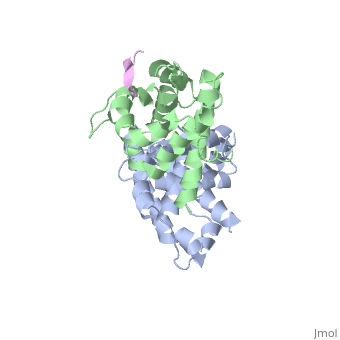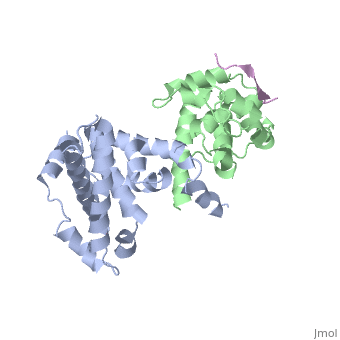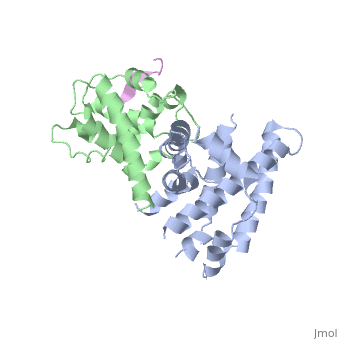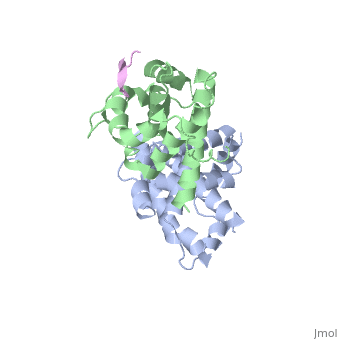
RB POCKET BOUND TO E7 LXCXE MOTIF
Contents |
Overview
The pocket domain of the retinoblastoma (Rb) tumour suppressor is central, to Rb function, and is frequently inactivated by the binding of the human, papilloma virus E7 oncoprotein in cervical cancer. The crystal structure, of the Rb pocket bound to a nine-residue E7 peptide containing the LxCxE, motif, shared by other Rb-binding viral and cellular proteins, shows that, the LxCxE peptide binds a highly conserved groove on the B-box portion of, the pocket; the A-box portion appears to be required for the stable, folding of the B box. Also highly conserved is the extensive A-B, interface, suggesting that it may be an additional protein-binding site., The A and B boxes each contain the cyclin-fold structural motif, with the, LxCxE-binding site on the B-box cyclin fold being similar to a, Cdk2-binding site of cyclin A and to a TBP-binding site of TFIIB.
Disease
Known diseases associated with this structure: Bladder cancer OMIM:[180200], Osteosarcoma OMIM:[180200], Pinealoma with bilateral retinoblastoma OMIM:[180200], Retinoblastoma OMIM:[180200]
About this Structure
1GUX is a Protein complex structure of sequences from Homo sapiens and Human papillomavirus. Full crystallographic information is available from OCA.
Reference
Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7., Lee JO, Russo AA, Pavletich NP, Nature. 1998 Feb 26;391(6670):859-65. PMID:9495340
Page seeded by OCA on Mon Nov 12 17:09:25 2007