Sandbox reserved 1752
From Proteopedia
| Line 70: | Line 70: | ||
== UvrD Motifs == | == UvrD Motifs == | ||
There are 7 sequence motifs and a Q motif conserved among the SF1 and the SF2. There are 4 domains that these motifs fit into (not shown). The domains are 1A, 1B, 2A, and 2B.I, Ia, II-VI are involved in ATP binding. Motifs Ia, III, and V are involved in ssDNA binding. Motif IV is reported to be unique in SF1. They found in their paper, seven new sequence motifs conserved among UvrD homologs. They are Ib, Ic, Id, IVb, IVc, Va, and VIa. These conserved residues are involved in DNA binding or domain 1B and 2B interactions [1]. | There are 7 sequence motifs and a Q motif conserved among the SF1 and the SF2. There are 4 domains that these motifs fit into (not shown). The domains are 1A, 1B, 2A, and 2B.I, Ia, II-VI are involved in ATP binding. Motifs Ia, III, and V are involved in ssDNA binding. Motif IV is reported to be unique in SF1. They found in their paper, seven new sequence motifs conserved among UvrD homologs. They are Ib, Ic, Id, IVb, IVc, Va, and VIa. These conserved residues are involved in DNA binding or domain 1B and 2B interactions [1]. | ||
| - | In total, there are<scene name='92/925553/Uvrd_labeled_motifs_complete/3'> 16 binding motifs</scene> for UvrD, which are conserved in other homologous structures, such as PcrA, Rep, and Srs2. The homologous structures mentioned are Helicase 2 homologs, which appear in different species. These conserved motifs are important to maintain the function of <ref name="ATP Binding" | + | In total, there are<scene name='92/925553/Uvrd_labeled_motifs_complete/3'> 16 binding motifs</scene> for UvrD, which are conserved in other homologous structures, such as PcrA, Rep, and Srs2. The homologous structures mentioned are Helicase 2 homologs, which appear in different species. These conserved [[motifs]] are important to maintain the function of<ref name="ATP Binding">UvrD. |
== Separation Pin == | == Separation Pin == | ||
The "<scene name='92/925553/Pin_complex/1'>separation pin</scene>" is a part of the 2B domain and is responsible for unwinding the DNA. This uses a 2 step power stroke, one stroke when ATP is bound and another stroke when ADP and P<sub>i</sub> are released. The GIG motif and separation pin work together to unwind the DNA and move it out of the way so UvrD can unwind more DNA. The separation pin also prevents ssDNA once unwound from moving backwards and from reannealing. The proposed method is called the wrench-and-inchworm method, which is when the enzyme binds DNA and attaches at different points and then moves 1 nucleotide per ATP molecule.After an ATP molecule is released, UvrD is then ready to proceed forward to the next nucleotide [1]. | The "<scene name='92/925553/Pin_complex/1'>separation pin</scene>" is a part of the 2B domain and is responsible for unwinding the DNA. This uses a 2 step power stroke, one stroke when ATP is bound and another stroke when ADP and P<sub>i</sub> are released. The GIG motif and separation pin work together to unwind the DNA and move it out of the way so UvrD can unwind more DNA. The separation pin also prevents ssDNA once unwound from moving backwards and from reannealing. The proposed method is called the wrench-and-inchworm method, which is when the enzyme binds DNA and attaches at different points and then moves 1 nucleotide per ATP molecule.After an ATP molecule is released, UvrD is then ready to proceed forward to the next nucleotide [1]. | ||
Revision as of 01:16, 12 October 2022
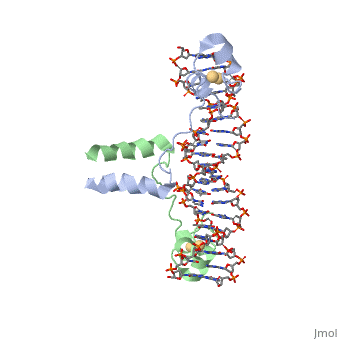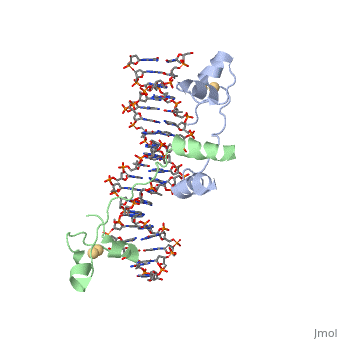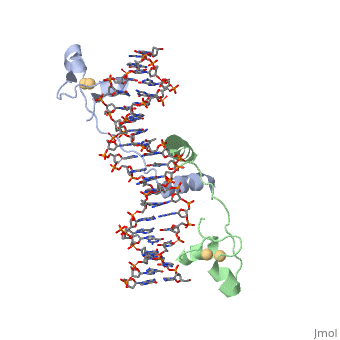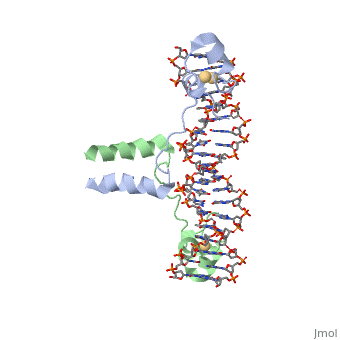
==DNA RECOGNITION BY GAL4: STRUCTURE OF A PROTEIN/DNA COMPLEX==
| |||||||||||
UvrD
, also known as Helicase II, is one of many components responsible in repairing DNA damage. Helicases use energy from ATP to unwind double helices in metabolic pathways using nucleic acids. ATP molecules are typically used to store energy shared between phosphate groups that gets released when breaking bonds to drive catabolic reactions.
Helicases were found in the 1970’s to be DNA-dependent ATPases, meaning that they use ATP hydrolysis to complete its interactions with the different types of nucleic acids it comes into contact with. Helicase II, also called UvrD is the founding member of SF1, one group of six superfamiliies used to identify helicases. SF1 and SF2 members share seven conserved sequence motifs that are involved in ATP Binding [1]. UvrD is important in replication, recombination, and repair from ultraviolet damage and mismatched base pairs. Nucleotide excision repair in a normal cell is supposed to correct pyrimidine dimers and other DNA lesions when bases are displaced from their normal positions. UvrD pairs up with the UvrABC endonuclease system, which works to displace the DNA. This is then repaired by PolI and DNA ligase [3]. A sub pathway of nucleotide excision repair is transcription-coupled repair, which works with an RNA polymerase to make repairs to damage to DNA [2].
UvrD Motifs
There are 7 sequence motifs and a Q motif conserved among the SF1 and the SF2. There are 4 domains that these motifs fit into (not shown). The domains are 1A, 1B, 2A, and 2B.I, Ia, II-VI are involved in ATP binding. Motifs Ia, III, and V are involved in ssDNA binding. Motif IV is reported to be unique in SF1. They found in their paper, seven new sequence motifs conserved among UvrD homologs. They are Ib, Ic, Id, IVb, IVc, Va, and VIa. These conserved residues are involved in DNA binding or domain 1B and 2B interactions [1]. In total, there are for UvrD, which are conserved in other homologous structures, such as PcrA, Rep, and Srs2. The homologous structures mentioned are Helicase 2 homologs, which appear in different species. These conserved motifs are important to maintain the function of[2]