We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Cell division protein
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
== Structure and Biochemistry of FtsZ == | == Structure and Biochemistry of FtsZ == | ||
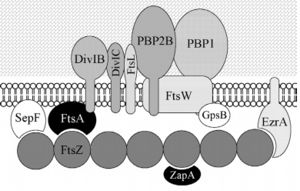
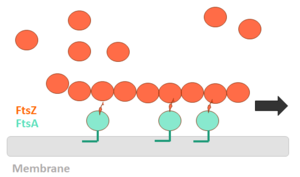
| - | FtsZ is a tubulin-like protein, which is widely conserved in bacteria and the main component of the bacterial cytokinesis machine, or “divisome.” FtsZ is a 40 kDa protein that folds into two independent globular domains [<scene name='81/817988/N-terminal_domain/1'>N-terminal</scene> (1-203) and <scene name='81/817988/C-terminal_domain/1'>C-terminal</scene> (204-316)] and has an unstructured tail of about 50 amino acids followed by a 15–17 conserved amino acid sequence at its extreme C-terminus. This conserved terminal sequence is also known as the ‘C-terminal peptide’ (CTP), since it is in the N-terminal domain that the nucleotide binding region is contained. Self-assembly of FtsZ involves interactions between the C-terminal globular domain of one subunit with the N-terminal globular domain of another subunit. The CTP, on the other hand, is the binding site for several of the proteins that interact with FtsZ. The N-terminal and C-terminal domain are separated by the central <scene name='81/817988/H7helix/1'>H7 helix</scene> (178-202). | + | '''FtsZ''' is a tubulin-like protein, which is widely conserved in bacteria and the main component of the bacterial cytokinesis machine, or “divisome.” FtsZ is a 40 kDa protein that folds into two independent globular domains [<scene name='81/817988/N-terminal_domain/1'>N-terminal</scene> (1-203) and <scene name='81/817988/C-terminal_domain/1'>C-terminal</scene> (204-316)] and has an unstructured tail of about 50 amino acids followed by a 15–17 conserved amino acid sequence at its extreme C-terminus. This conserved terminal sequence is also known as the ‘C-terminal peptide’ (CTP), since it is in the N-terminal domain that the nucleotide binding region is contained. Self-assembly of FtsZ involves interactions between the C-terminal globular domain of one subunit with the N-terminal globular domain of another subunit. The CTP, on the other hand, is the binding site for several of the proteins that interact with FtsZ. The N-terminal and C-terminal domain are separated by the central <scene name='81/817988/H7helix/1'>H7 helix</scene> (178-202). |
FtsZ and tubulin share several essential properties: their assembly is cooperative, stimulated by GTP, and leads to GTP hydrolysis; they form dynamic polymers whose turnover is dependent on nucleotide hydrolysis; they use essentially the same bond for polymer formation; and recent evidence indicates that they undergo similar allosteric transitions upon polymerization. The folding of the FtsZ N-terminal domain is typical of GTPases, with six parallel β-strands (S1-S6) surrounded by six α-helices (H1-H6), named according to the tubulin structure (show <scene name='81/817988/Secondarystructure/2'>secondary structure</scene>). The C-terminal domain is formed by four parallel β-strands (S7-S10) surrounded by two helices, with the antiparallel strand S10. The residues in the T1-T4 loops make contact with the phosphate groups of the GDP. The T5 loop between S5 and H5 helix contains residues that make hydrogen bonds with the sugar moiety and also contacts with the phosphate of GDP, while interactions with the nucleotide nitrogen base are done by residues of the H7 helix. | FtsZ and tubulin share several essential properties: their assembly is cooperative, stimulated by GTP, and leads to GTP hydrolysis; they form dynamic polymers whose turnover is dependent on nucleotide hydrolysis; they use essentially the same bond for polymer formation; and recent evidence indicates that they undergo similar allosteric transitions upon polymerization. The folding of the FtsZ N-terminal domain is typical of GTPases, with six parallel β-strands (S1-S6) surrounded by six α-helices (H1-H6), named according to the tubulin structure (show <scene name='81/817988/Secondarystructure/2'>secondary structure</scene>). The C-terminal domain is formed by four parallel β-strands (S7-S10) surrounded by two helices, with the antiparallel strand S10. The residues in the T1-T4 loops make contact with the phosphate groups of the GDP. The T5 loop between S5 and H5 helix contains residues that make hydrogen bonds with the sugar moiety and also contacts with the phosphate of GDP, while interactions with the nucleotide nitrogen base are done by residues of the H7 helix. | ||
Revision as of 10:00, 16 November 2022
| |||||||||||
References
[1] [2] [3] [4] [5] [6] [7] [8]
- ↑ Bisson-Filho AW, Discola KF, Castellen P, Blasios V, Martins A, Sforca ML, Garcia W, Zeri AC, Erickson HP, Dessen A, Gueiros-Filho FJ. FtsZ filament capping by MciZ, a developmental regulator of bacterial division. Proc Natl Acad Sci U S A. 2015 Apr 6. pii: 201414242. PMID:25848052 doi:http://dx.doi.org/10.1073/pnas.1414242112
- ↑ Wang, X. & Lutkenhaus, J. FtsZ ring: the eubacterial division apparatus conserved in archaebacteria.Mol. Microbiol. 21, 313–319 (1996). Gueiros-Filho, F. J. & Losick, R. A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev. 16, 2544–2556 (2002).
- ↑ Wang, X., Huang, J., Mukherjee, A., Cao, C., and Lutkenhaus, J. (1997). Analysis of the interaction of FtsZ with itself, GTP, and FtsA. J. Bacteriol. 179, 5551–5559.
- ↑ Vaughan S, Wickstead B, Gull K, Addinall SG. Molecular evolution of FtsZ protein sequences encoded within the genomes of archaea, bacteria, and eukaryota. J Mol Evol. 2004 Jan;58(1):19-29. doi: 10.1007/s00239-003-2523-5. PMID:14743312 doi:http://dx.doi.org/10.1007/s00239-003-2523-5
- ↑ Szwedziak P, Wang Q, Bharat TA, Tsim M, Lowe J. Architecture of the ring formed by the tubulin homologue FtsZ in bacterial cell division. Elife. 2014 Dec 9;3:e04601. doi: 10.7554/eLife.04601. PMID:25490152 doi:http://dx.doi.org/10.7554/eLife.04601
- ↑ Huecas, S. et al. Energetics and geometry of FtsZ polymers: nucleated self-assembly of single protofilaments. Biophys. J. 94, 1796–1806 (2008).
- ↑ Lan, G. et al. (2009) Condensation of FtsZ filaments can drive bacterial cell division. Proc. Natl. Acad. Sci. U. S. A. 106, 121–126
- ↑ FILHO, Frederico Gueiros. Cell Division. In: GRAUMANN, Peter L. et al. Bacillus: Cellular and Molecular Biology. Germany: Caister Academic Press, 2017.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Jonathan Cardoso C. Vieira, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky