We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
4iou
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
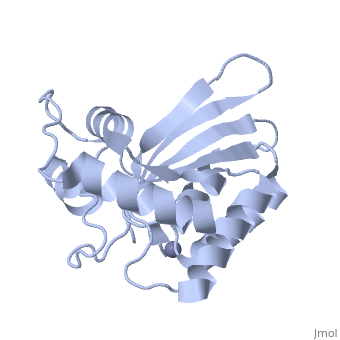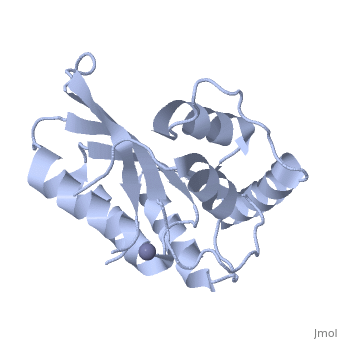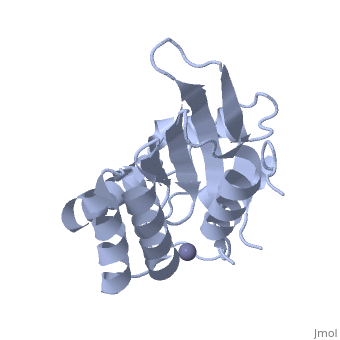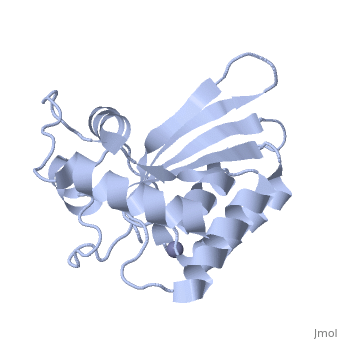
<StructureSection load='4iou' size='340' side='right'caption='[[4iou]], [[Resolution|resolution]] 2.75Å' scene=''> | <StructureSection load='4iou' size='340' side='right'caption='[[4iou]], [[Resolution|resolution]] 2.75Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
| - | <table><tr><td colspan='2'>[[4iou]] is a 4 chain structure with sequence from [ | + | <table><tr><td colspan='2'>[[4iou]] is a 4 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=4IOU OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=4IOU FirstGlance]. <br> |
| - | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=ZN:ZINC+ION'>ZN</scene> | + | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> |
| - | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=4iou FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=4iou OCA], [https://pdbe.org/4iou PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=4iou RCSB], [https://www.ebi.ac.uk/pdbsum/4iou PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=4iou ProSAT]</span></td></tr> | |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | + | |
</table> | </table> | ||
== Function == | == Function == | ||
| - | [ | + | [https://www.uniprot.org/uniprot/ABC3F_HUMAN ABC3F_HUMAN] DNA deaminase (cytidine deaminase) which acts as an inhibitor of retrovirus replication and retrotransposon mobility via deaminase-dependent and -independent mechanisms. Exhibits antiviral activity against vif-deficient HIV-1. After the penetration of retroviral nucleocapsids into target cells of infection and the initiation of reverse transcription, it can induce the conversion of cytosine to uracil in the minus-sense single-strand viral DNA, leading to G-to-A hypermutations in the subsequent plus-strand viral DNA. The resultant detrimental levels of mutations in the proviral genome, along with a deamination-independent mechanism that works prior to the proviral integration, together exert efficient antiretroviral effects in infected target cells. Selectively targets single-stranded DNA and does not deaminate double-stranded DNA or single- or double-stranded RNA. Exhibits antiviral activity also against hepatitis B virus (HBV), equine infectious anemia virus (EIAV), xenotropic MuLV-related virus (XMRV) and simian foamy virus (SFV) and may inhibit the mobility of LTR and non-LTR retrotransposons. May also play a role in the epigenetic regulation of gene expression through the process of active DNA demethylation.<ref>PMID:15152192</ref> <ref>PMID:16527742</ref> <ref>PMID:16378963</ref> <ref>PMID:19458006</ref> <ref>PMID:20219927</ref> <ref>PMID:20335265</ref> <ref>PMID:20062055</ref> <ref>PMID:21496894</ref> <ref>PMID:21835787</ref> <ref>PMID:22915799</ref> <ref>PMID:22807680</ref> <ref>PMID:23097438</ref> <ref>PMID:23152537</ref> |
<div style="background-color:#fffaf0;"> | <div style="background-color:#fffaf0;"> | ||
== Publication Abstract from PubMed == | == Publication Abstract from PubMed == | ||
| Line 23: | Line 22: | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
| - | [[Category: | + | [[Category: Homo sapiens]] |
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
| - | [[Category: Bohn | + | [[Category: Bohn M]] |
| - | [[Category: Schiffer | + | [[Category: Schiffer CA]] |
| - | [[Category: Shandilya | + | [[Category: Shandilya SMD]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 21:00, 16 November 2022
Crystal structure of the HIV-1 Vif binding, catalytically active domain of APOBEC3F
| |||||||||||