We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Androgen receptor
From Proteopedia
(Difference between revisions)
| Line 104: | Line 104: | ||
'''Urinary incontinence''' denotes involuntary bladder urine leakage amongst women commonly with decreased pelvic muscle strength. As the pelvic floor muscles contain high levels of AR, it is a relevant target for SARM therapy <ref name="Steroids" />. | '''Urinary incontinence''' denotes involuntary bladder urine leakage amongst women commonly with decreased pelvic muscle strength. As the pelvic floor muscles contain high levels of AR, it is a relevant target for SARM therapy <ref name="Steroids" />. | ||
====Side effects of SARMs==== | ====Side effects of SARMs==== | ||
| - | Despite the consistent effect demonstrated by SARMs on lean body mass accrual, reductions in high-density lipoprotein (HDL) seem to be an important concern with these compounds, | + | Despite the consistent effect demonstrated by SARMs on lean body mass accrual, reductions in high-density lipoprotein (HDL) seem to be an important concern with these compounds, although it occurs to a lesser extent compared to testosterone <ref name="clinical trials">PMID: 32476495</ref>. |
| - | SARMs administration has also been related to hepatotoxicity and some compounds have | + | SARMs administration has also been related to hepatotoxicity and some compounds have been linked to alterations in liver enzymes. The most common adverse events are an increase in alanine transaminase and aspartate transaminase <ref name="clinical trials" />. |
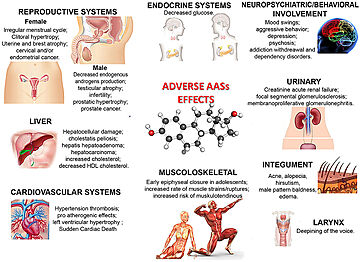
The anabolic effects of SARMs and their lack of androgenic side effects have made them of great interest to the bodybuilding community and create the potential for abuse among competitive athletes <ref name="SARMs knowledge" />. | The anabolic effects of SARMs and their lack of androgenic side effects have made them of great interest to the bodybuilding community and create the potential for abuse among competitive athletes <ref name="SARMs knowledge" />. | ||
| Line 111: | Line 111: | ||
===Antagonist=== | ===Antagonist=== | ||
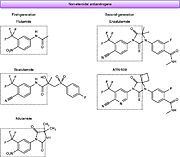
'''[[Image:Non-steroideal anti-androgens.jpeg | thumb | right | Reproduced from Helsen et al. <ref name="ARA prostate" />]]''' | '''[[Image:Non-steroideal anti-androgens.jpeg | thumb | right | Reproduced from Helsen et al. <ref name="ARA prostate" />]]''' | ||
| - | These kinds of drugs were developed with the objective to avoid the side effects associated with cross reactivity of steroidal ARA, increasing the selectivity and the affinity to the AR, limiting the association with other steroids nuclear receptors <ref name="bicalutamide" />. Also, their non-steroidal structure improved oral bioavailability | + | These kinds of drugs were developed with the objective to avoid the side effects associated with cross reactivity of steroidal ARA, increasing the selectivity and the affinity to the AR, limiting the association with other steroids nuclear receptors <ref name="bicalutamide" />. Also, their non-steroidal structure improved oral bioavailability what constitutes another advantage in comparison with steroidal ARA <ref name="bicalutamide" />. Some examples are flutamide, bicalutamide <ref name="ARA prostate" /><ref name="bicalutamide" /><ref name="nonsteroidal" /><ref name="Bicalutamide functions">PMID: 12015321</ref><ref name="Unexpected">PMID: 21506597</ref><ref name="Role of AR">PMID: 30209899</ref> or apalutamide (ARN-509) <ref name="ARA prostate" /><ref name="Role of AR" />. |
====Bicalutamide==== | ====Bicalutamide==== | ||
<scene name='54/543362/Bicalutamide_in_ar/3'>R-Bicalutamide</scene>, marketed as Casodex <ref name="ARA prostate" /><ref name="nonsteroidal" />, is one of the most stable and tolerated ARA used in the treatment of prostate cancer <ref name="ARA prostate" /><ref name="bicalutamide" /><ref name="AAWS">PMID: 28971898</ref>, belonging to the first generation of antiandrogens developed <ref name="ARA prostate" /><ref name="MoA">PMID: 35245614</ref>. | <scene name='54/543362/Bicalutamide_in_ar/3'>R-Bicalutamide</scene>, marketed as Casodex <ref name="ARA prostate" /><ref name="nonsteroidal" />, is one of the most stable and tolerated ARA used in the treatment of prostate cancer <ref name="ARA prostate" /><ref name="bicalutamide" /><ref name="AAWS">PMID: 28971898</ref>, belonging to the first generation of antiandrogens developed <ref name="ARA prostate" /><ref name="MoA">PMID: 35245614</ref>. | ||
| - | It is a competitive antagonist <ref name="Bicalutamide functions" /><ref name="MoA" /><ref name="AAWS" /> which binds to the LBD producing a transcriptionally inactive AR <ref name="Bicalutamide functions" />. However, it seems that the long-term use of these drugs and other first generation antiandrogens lead to withdrawal syndrome in prostate cancer resistant to castration patients <ref name="ARA prostate" /><ref name="nonsteroidal" />. In many cases associated AR mutations, like W741L, can switch the mechanism of action of the drug from antagonist to agonist or partial agonist <ref name="ARA prostate" /><ref name="bicalutamide" /><ref name="nonsteroidal" /><ref name="Unexpected" /><ref name="MoA" />. | + | It is a competitive antagonist <ref name="Bicalutamide functions" /><ref name="MoA" /><ref name="AAWS" /> which binds to the LBD producing a transcriptionally inactive AR <ref name="Bicalutamide functions" />. However, it seems that the long-term use of these drugs and other first generation antiandrogens lead to withdrawal syndrome in prostate cancer resistant to castration patients <ref name="ARA prostate" /><ref name="nonsteroidal" />. In many cases, associated AR mutations, like W741L, can switch the mechanism of action of the drug from antagonist to agonist or partial agonist <ref name="ARA prostate" /><ref name="bicalutamide" /><ref name="nonsteroidal" /><ref name="Unexpected" /><ref name="MoA" />. |
| - | Although bicalutamide has been patented since 1982 and approved to be clinical used by the FDA since 1995 <ref name="bicalutamide" />, its mechanism of action | + | Although bicalutamide has been patented since 1982 and approved to be clinical used by the FDA since 1995 <ref name="bicalutamide" />, its mechanism of action is still under debate. The X-ray structure of the wild-type AR bound to an antagonist is not yet solved <ref name="MoA" />. Changes in the conformation of the receptor, due to association with antagonists, have been hypothesized to be similar to those produced in the steroid receptor family <ref name="ARA prostate" /><ref name="MoA" />. |
| - | When an agonist or a ligand binds to the LBD, it seems to induce a conformation of the steroid receptor which makes H12 closes off the pocket of LBD allowing the | + | When an agonist or a ligand binds to the LBD, it seems to induce a conformation of the steroid receptor which makes H12 closes off the pocket of LBD allowing the binding of cofactors. That permits the steroid receptor function allowing the DNA transcription <ref name="ARA prostate" />. |
| - | Although, when an antagonist is | + | Although, when an antagonist is bound, H12 seems to be more separated to the LBD, disabling the binding of coactivators <ref name="ARA prostate" /> and the migration of the nuclear receptor into the nucleus <ref name="MoA" />. |
| - | Nonetheless, the AR has some structural singularities that may not let this change of conformation. One of the most important changes is the additional C-terminal region in H12 anchored to the receptor by the formation of a ß-sheet, limiting its movement <ref name="ARA prostate" /><ref name="MoA" />. Due to this structural difference, ''in silico'' approaches have suggested that the antiandrogen effect of bicalutamide may be produced by the instability of the homodimer <ref name="MoA" />. That may lend to the | + | Nonetheless, the AR has some structural singularities that may not let this change of conformation. One of the most important changes is the additional C-terminal region in H12 anchored to the receptor by the formation of a ß-sheet, limiting its movement <ref name="ARA prostate" /><ref name="MoA" />. Due to this structural difference, ''in silico'' approaches have suggested that the antiandrogen effect of bicalutamide may be produced by the instability of the homodimer <ref name="MoA" />. That may lend to the dissociation of the homodimer preventing the transcriptional activity of the AR and explaining the mechanism of action of this drug <ref name="MoA" />. In addition,'' in silico'' analysis have shown that the W741L mutation leads to a more stable bicalutamide-AR homodimer, which may provide some insight into the withdrawal syndrome observed in bicalutamide treatment <ref name="MoA" />. |
| - | For future research, it will be useful to understand the | + | For future research, it will be useful to understand the precise mechanism of action of antiandrogens currently used in the clinic, with the objective of developing new drugs which can escape from the antagonist-agonist switch seen in bicalutamide or flutamide. One example of this is apalutamide, a non-steroidal second generation antiandrogen <ref name="ARA prostate" /><ref name="Role of AR" />, approved for use in non metastatic castration resistant prostate cancer patients by the FDA in 2018 <ref name="Role of AR" />. See also the [https://clinicaltrials.gov/ct2/show/NCT01946204 SPARTAN study]<ref>PMID: 29420164</ref>. This new drug has promising uses but it is still associated with side effects like an increased level of falls in patients with the treatment vs placebo <ref>PMID: 36209239</ref>. |
Revision as of 09:43, 29 November 2022
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Davey RA, Grossmann M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin Biochem Rev. 2016 Feb;37(1):3-15. PMID:27057074
- ↑ Kolyvas EA, Caldas C, Kelly K, Ahmad SS. Androgen receptor function and targeted therapeutics across breast cancer subtypes. Breast Cancer Res. 2022 Nov 14;24(1):79. doi: 10.1186/s13058-022-01574-4. PMID:36376977 doi:http://dx.doi.org/10.1186/s13058-022-01574-4
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 Tan MH, Li J, Xu HE, Melcher K, Yong EL. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin. 2015 Jan;36(1):3-23. doi: 10.1038/aps.2014.18. Epub 2014 Jun , 9. PMID:24909511 doi:http://dx.doi.org/10.1038/aps.2014.18
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Messner EA, Steele TM, Tsamouri MM, Hejazi N, Gao AC, Mudryj M, Ghosh PM. The Androgen Receptor in Prostate Cancer: Effect of Structure, Ligands and Spliced Variants on Therapy. Biomedicines. 2020 Oct 15;8(10):422. doi: 10.3390/biomedicines8100422. PMID:33076388 doi:http://dx.doi.org/10.3390/biomedicines8100422
- ↑ van Royen ME, van Cappellen WA, de Vos C, Houtsmuller AB, Trapman J. Stepwise androgen receptor dimerization. J Cell Sci. 2012 Apr 15;125(Pt 8):1970-9. doi: 10.1242/jcs.096792. Epub 2012 Feb , 10. PMID:22328501 doi:http://dx.doi.org/10.1242/jcs.096792
- ↑ Kono M, Fujii T, Lim B, Karuturi MS, Tripathy D, Ueno NT. Androgen Receptor Function and Androgen Receptor-Targeted Therapies in Breast Cancer: A Review. JAMA Oncol. 2017 Sep 1;3(9):1266-1273. doi: 10.1001/jamaoncol.2016.4975. PMID:28301631 doi:http://dx.doi.org/10.1001/jamaoncol.2016.4975
- ↑ 7.0 7.1 7.2 Gibson DA, Saunders PTK, McEwan IJ. Androgens and androgen receptor: Above and beyond. Mol Cell Endocrinol. 2018 Apr 15;465:1-3. doi: 10.1016/j.mce.2018.02.013. Epub , 2018 Feb 24. PMID:29481861 doi:http://dx.doi.org/10.1016/j.mce.2018.02.013
- ↑ Ceruti JM, Leiros GJ, Balana ME. Androgens and androgen receptor action in skin and hair follicles. Mol Cell Endocrinol. 2018 Apr 15;465:122-133. doi: 10.1016/j.mce.2017.09.009. , Epub 2017 Sep 12. PMID:28912032 doi:http://dx.doi.org/10.1016/j.mce.2017.09.009
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Solomon ZJ, Mirabal JR, Mazur DJ, Kohn TP, Lipshultz LI, Pastuszak AW. Selective Androgen Receptor Modulators: Current Knowledge and Clinical Applications. Sex Med Rev. 2019 Jan;7(1):84-94. doi: 10.1016/j.sxmr.2018.09.006. Epub 2018 Nov , 30. PMID:30503797 doi:http://dx.doi.org/10.1016/j.sxmr.2018.09.006
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 Burris TP, Solt LA, Wang Y, Crumbley C, Banerjee S, Griffett K, Lundasen T, Hughes T, Kojetin DJ. Nuclear receptors and their selective pharmacologic modulators. Pharmacol Rev. 2013 Mar 1;65(2):710-78. doi: 10.1124/pr.112.006833. Print 2013 , Apr. PMID:23457206 doi:http://dx.doi.org/10.1124/pr.112.006833
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 Christiansen AR, Lipshultz LI, Hotaling JM, Pastuszak AW. Selective androgen receptor modulators: the future of androgen therapy? Transl Androl Urol. 2020 Mar;9(Suppl 2):S135-S148. doi: 10.21037/tau.2019.11.02. PMID:32257854 doi:http://dx.doi.org/10.21037/tau.2019.11.02
- ↑ 12.0 12.1 12.2 12.3 Narayanan R, Coss CC, Dalton JT. Development of selective androgen receptor modulators (SARMs). Mol Cell Endocrinol. 2018 Apr 15;465:134-142. doi: 10.1016/j.mce.2017.06.013. , Epub 2017 Jun 15. PMID:28624515 doi:http://dx.doi.org/10.1016/j.mce.2017.06.013
- ↑ Culig Z, Klocker H, Bartsch G, Hobisch A. Androgen receptors in prostate cancer. Endocr Relat Cancer. 2002 Sep;9(3):155-70. doi: 10.1677/erc.0.0090155. PMID:12237244 doi:http://dx.doi.org/10.1677/erc.0.0090155
- ↑ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 14.10 14.11 14.12 14.13 14.14 14.15 14.16 14.17 14.18 Helsen C, Van den Broeck T, Voet A, Prekovic S, Van Poppel H, Joniau S, Claessens F. Androgen receptor antagonists for prostate cancer therapy. Endocr Relat Cancer. 2014 Aug;21(4):T105-18. doi: 10.1530/ERC-13-0545. Epub 2014 , Mar 17. PMID:24639562 doi:http://dx.doi.org/10.1530/ERC-13-0545
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 Sesti F, Pofi R, Minnetti M, Tenuta M, Gianfrilli D, Isidori AM. Late-onset hypogonadism: Reductio ad absurdum of the cardiovascular risk-benefit of testosterone replacement therapy. Andrology. 2020 Nov;8(6):1614-1627. doi: 10.1111/andr.12876. Epub 2020 Aug 11. PMID:32737921 doi:http://dx.doi.org/10.1111/andr.12876
- ↑ 16.0 16.1 16.2 16.3 16.4 Kaur H, Werstuck GH. The Effect of Testosterone on Cardiovascular Disease and Cardiovascular Risk Factors in Men: A Review of Clinical and Preclinical Data. CJC Open. 2021 May 17;3(10):1238-1248. doi: 10.1016/j.cjco.2021.05.007. , eCollection 2021 Oct. PMID:34888506 doi:http://dx.doi.org/10.1016/j.cjco.2021.05.007
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 Bohl CE, Gao W, Miller DD, Bell CE, Dalton JT. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc Natl Acad Sci U S A. 2005 Apr 26;102(17):6201-6. Epub 2005 Apr 15. PMID:15833816
- ↑ 18.0 18.1 18.2 18.3 18.4 Gao W, Kim J, Dalton JT. Pharmacokinetics and pharmacodynamics of nonsteroidal androgen receptor ligands. Pharm Res. 2006 Aug;23(8):1641-58. doi: 10.1007/s11095-006-9024-3. PMID:16841196 doi:http://dx.doi.org/10.1007/s11095-006-9024-3
- ↑ Sessa F, Salerno M, Di Mizio G, Bertozzi G, Messina G, Tomaiuolo B, Pisanelli D, Maglietta F, Ricci P, Pomara C. Anabolic Androgenic Steroids: Searching New Molecular Biomarkers. Front Pharmacol. 2018 Nov 20;9:1321. doi: 10.3389/fphar.2018.01321. eCollection , 2018. PMID:30524281 doi:http://dx.doi.org/10.3389/fphar.2018.01321
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 Machek SB, Cardaci TD, Wilburn DT, Willoughby DS. Considerations, possible contraindications, and potential mechanisms for deleterious effect in recreational and athletic use of selective androgen receptor modulators (SARMs) in lieu of anabolic androgenic steroids: A narrative review. Steroids. 2020 Dec;164:108753. doi: 10.1016/j.steroids.2020.108753. Epub 2020 Oct , 24. PMID:33148520 doi:http://dx.doi.org/10.1016/j.steroids.2020.108753
- ↑ 21.0 21.1 Fonseca GWPD, Dworatzek E, Ebner N, Von Haehling S. Selective androgen receptor modulators (SARMs) as pharmacological treatment for muscle wasting in ongoing clinical trials. Expert Opin Investig Drugs. 2020 Aug;29(8):881-891. doi: , 10.1080/13543784.2020.1777275. Epub 2020 Jun 18. PMID:32476495 doi:http://dx.doi.org/10.1080/13543784.2020.1777275
- ↑ 22.0 22.1 22.2 Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J Biol Chem. 2002 Jul 19;277(29):26321-6. doi: 10.1074/jbc.M203310200. Epub 2002 , May 15. PMID:12015321 doi:http://dx.doi.org/10.1074/jbc.M203310200
- ↑ 23.0 23.1 Duke CB, Jones A, Bohl CE, Dalton JT, Miller DD. Unexpected Binding Orientation of Bulky-B-Ring Anti-Androgens and Implications for Future Drug Targets. J Med Chem. 2011 Apr 20. PMID:21506597 doi:10.1021/jm2000097
- ↑ 24.0 24.1 24.2 24.3 Fujita K, Nonomura N. Role of Androgen Receptor in Prostate Cancer: A Review. World J Mens Health. 2019 Sep;37(3):288-295. doi: 10.5534/wjmh.180040. Epub 2018 , Sep 10. PMID:30209899 doi:http://dx.doi.org/10.5534/wjmh.180040
- ↑ 25.0 25.1 Leone G, Tucci M, Buttigliero C, Zichi C, Pignataro D, Bironzo P, Vignani F, Scagliotti GV, Di Maio M. Antiandrogen withdrawal syndrome (AAWS) in the treatment of patients with prostate cancer. Endocr Relat Cancer. 2018 Jan;25(1):R1-R9. doi: 10.1530/ERC-17-0355. Epub 2017 , Sep 28. PMID:28971898 doi:http://dx.doi.org/10.1530/ERC-17-0355
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 26.6 26.7 26.8 26.9 Cavaliere F, Cozzini P. An insight about the mechanism of action (MoA) of R-bicalutamide on the androgen receptor homodimer using molecular dynamic. Toxicol Appl Pharmacol. 2022 Apr 1;440:115953. doi: 10.1016/j.taap.2022.115953. , Epub 2022 Mar 1. PMID:35245614 doi:http://dx.doi.org/10.1016/j.taap.2022.115953
- ↑ Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H, Lopez-Gitlitz A, Trudel GC, Espina BM, Shu Y, Park YC, Rackoff WR, Yu MK, Small EJ. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018 Apr 12;378(15):1408-1418. doi: 10.1056/NEJMoa1715546. Epub , 2018 Feb 8. PMID:29420164 doi:http://dx.doi.org/10.1056/NEJMoa1715546
- ↑ Pollock Y, Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik B, Olmos D, Lee JY, Uemura H, Bhaumik A, Londhe A, Rooney B, Brookman-May SD, De Porre P, Mundle SD, Small EJ. Clinical characteristics associated with falls in patients with non-metastatic castration-resistant prostate cancer treated with apalutamide. Prostate Cancer Prostatic Dis. 2022 Oct 8. doi: 10.1038/s41391-022-00592-9. PMID:36209239 doi:http://dx.doi.org/10.1038/s41391-022-00592-9
Proteopedia Page Contributors and Editors (what is this?)
Cristina Benito, Michal Harel, Cristina Murga, Marta Roldan Lazaro, Alexander Berchansky, Joel L. Sussman, Wayne Decatur, David Sánchez Fernández