We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1761
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
The specific function of <scene name='93/934005/Spin/2'>Human ornithine aminotransferase</scene> (''h''OAT) is that of an enzyme. It can be found in humans, as well as mice and pigs. It helps transfer L-ornithine’s δ-amino group to α-ketoglutarate (α-KG). <ref>https://doi.org/10.1016/j.jbc.2022.101969</ref> There is a lack of understanding in regards to ''h''OAT's catalytic mechanism, even though it is a key component of human metabolism. As noted in the article, ''h''OAT operates on a "Bi-Bi, Ping-Pong" kinetic mechanism resulting in the first part of the reaction undergoing a conversion of PLP -> PMP and L-Orn -> L-GSA. Then PMP's amino group is catalyzed by ''h''OAT creating an α-KG. | The specific function of <scene name='93/934005/Spin/2'>Human ornithine aminotransferase</scene> (''h''OAT) is that of an enzyme. It can be found in humans, as well as mice and pigs. It helps transfer L-ornithine’s δ-amino group to α-ketoglutarate (α-KG). <ref>https://doi.org/10.1016/j.jbc.2022.101969</ref> There is a lack of understanding in regards to ''h''OAT's catalytic mechanism, even though it is a key component of human metabolism. As noted in the article, ''h''OAT operates on a "Bi-Bi, Ping-Pong" kinetic mechanism resulting in the first part of the reaction undergoing a conversion of PLP -> PMP and L-Orn -> L-GSA. Then PMP's amino group is catalyzed by ''h''OAT creating an α-KG. | ||
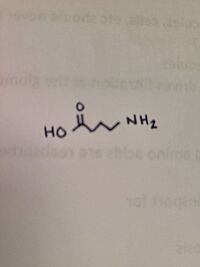
[[Image:Daba.jpg|200 px|thumb|right|DABA]] | [[Image:Daba.jpg|200 px|thumb|right|DABA]] | ||
| + | The binding affinities of GABA and AVA were higher and resulted in slower turnovers. This raised the question that AVA and GABA would be good drug targets, rather than specifically ''h''OAT. | ||
== Biological relevance and broader implications == | == Biological relevance and broader implications == | ||
| Line 13: | Line 14: | ||
== Important amino acids== | == Important amino acids== | ||
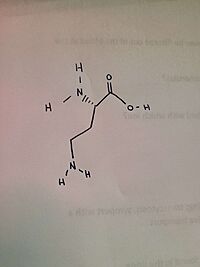
| - | The ligand of ''h''OAT is Pyridoxal-5'-Phosphate <scene name='93/934005/Plp/1'>(PLP)</scene>. The role of the catalytic amino acids in an enzyme is to bind to a substrate, changing the structure, causing bonds to break and new bonds to be formed. When there is a difficult reaction, the triad of amino acids works in tandem to facilitate the reaction. A <scene name='93/934005/Glu235/1'>Glu 235</scene>-<scene name='93/934005/Arg413/1'>Arg 413</scene> <scene name='93/934005/Arg_413_glu_235/1'>salt bridge</scene> was found on AVA, but not on GABA. | + | The ligand of ''h''OAT is Pyridoxal-5'-Phosphate <scene name='93/934005/Plp/1'>(PLP)</scene>. The role of the catalytic amino acids in an enzyme is to bind to a substrate, changing the structure, causing bonds to break and new bonds to be formed. When there is a difficult reaction, the triad of amino acids works in tandem to facilitate the reaction. A <scene name='93/934005/Glu235/1'>Glu 235</scene>-<scene name='93/934005/Arg413/1'>Arg 413</scene> <scene name='93/934005/Arg_413_glu_235/1'>salt bridge</scene> was found on AVA, but not on GABA. In regards to GABA, the salt bridge was disrupted in 30% of the monomers. |
| + | The crystal soaking experiments created an opportunity to decipher the reaction intermediates of the structures. While GABA linked to PLP covalently, PLP and catalytic Lys 292 were linked by AVA. | ||
== Structural highlights == | == Structural highlights == | ||
Revision as of 17:25, 13 December 2022
| This Sandbox is Reserved from November 4, 2022 through January 1, 2023 for use in the course CHEM 351 Biochemistry taught by Bonnie Hall at the Grand View University, Des Moines, USA. This reservation includes Sandbox Reserved 1755 through Sandbox Reserved 1764. |
To get started:
More help: Help:Editing |
Human ornithine aminotransferase (hOAT)
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ https://doi.org/10.1016/j.jbc.2022.101969
Butrin, A., Butrin, A., Wawrzak, Z., Moran, G. R., & Liu, D. (2022). Determination of the ph dependence, substrate specificity, and turnovers of alternative substrates for human ornithine aminotransferase. Journal of Biological Chemistry, 298(6), 101969. https://doi.org/10.1016/j.jbc.2022.101969