Dihydrodipicolinate synthase
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
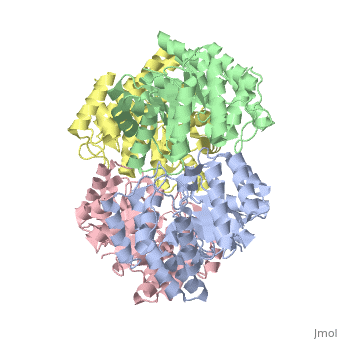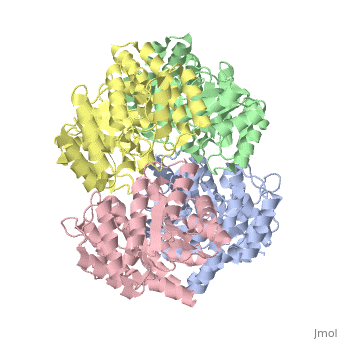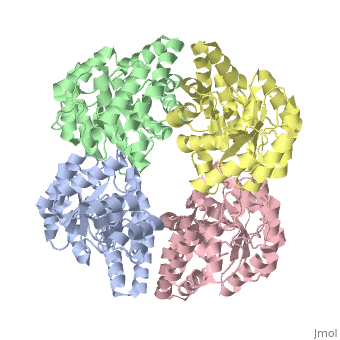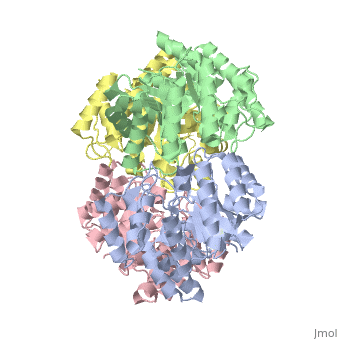
| - | One approach of the incorporation of genetic material from different species into bacterial genomes is infection by a bacteriophage. The ''yagE'' gene is located in the ''E. coli K12'' genome and it is part of prophage ''CP4-6'' encoding a 33-kDa putative dihydrodipicolinate synthase (DHDPS)-like protein (UniProtKB/Swiss-prot: [http://www.expasy.org/uniprot/P75682 P75682]). This DHDPS-like domain is a member of the N-acetyl neuraminate lyase (NAL) subfamily comprises an 8-fold <scene name='2v9d/Alpha_beta/2'>α/β-barrel</scene> ([http://en.wikipedia.org/wiki/TIM_barrel TIM barrel]) with a small C-terminal α-helical domain (Inter pro: [http://www.ebi.ac.uk/interpro/ISearch?query=IPR005263 IPR005263]). Many enzymes possess this DHDPS domain (''e.g.'' HBPHA, KDGA, NAL, DOGDH, and DHDPS). All these enzymes contain an 8-fold α/β barrel and a small C-terminal α-helical region. <scene name='2v8z/Comparison/2'>Comparison</scene> between <font color='red'><b>orthorhombic</b></font> ([[2v8z]]) and <font color='cyan'><b>monoclinic</b></font> ([[2v9d]]) crystal forms of YagE revealed striking similarity. | + | One approach of the incorporation of genetic material from different species into bacterial genomes is infection by a bacteriophage. The ''yagE'' gene is located in the ''E. coli K12'' genome and it is part of prophage ''CP4-6'' encoding a 33-kDa putative '''dihydrodipicolinate synthase''' or '''4-hydroxy-tetrahydrodihydrodipicolinate synthase (''DHDPS)-like protein (UniProtKB/Swiss-prot: [http://www.expasy.org/uniprot/P75682 P75682]). This DHDPS-like domain is a member of the N-acetyl neuraminate lyase (NAL) subfamily comprises an 8-fold <scene name='2v9d/Alpha_beta/2'>α/β-barrel</scene> ([http://en.wikipedia.org/wiki/TIM_barrel TIM barrel]) with a small C-terminal α-helical domain (Inter pro: [http://www.ebi.ac.uk/interpro/ISearch?query=IPR005263 IPR005263]). Many enzymes possess this DHDPS domain (''e.g.'' HBPHA, KDGA, NAL, DOGDH, and DHDPS). All these enzymes contain an 8-fold α/β barrel and a small C-terminal α-helical region. <scene name='2v8z/Comparison/2'>Comparison</scene> between <font color='red'><b>orthorhombic</b></font> ([[2v8z]]) and <font color='cyan'><b>monoclinic</b></font> ([[2v9d]]) crystal forms of YagE revealed striking similarity. |
{{Clear}} | {{Clear}} | ||
Revision as of 08:10, 22 December 2022
| |||||||||||
3D structures of dihydrodipicolinate synthase
Updated on 22-December-2022
Reference
Crystal structure of YagE, a putative DHDPS-like protein from Escherichia coli K12., Manicka S, Peleg Y, Unger T, Albeck S, Dym O, Greenblatt HM, Bourenkov G, Lamzin V, Krishnaswamy S, Sussman JL, Proteins. 2008 Jun;71(4):2102-8. PMID:18361457
- Created with the participation of Jaime Prilusky and Michal Harel.
Categories: Topic Page | Escherichia coli | Single protein | Albeck, S. | Bourenkov, G. | Dym, O. | Greenblatt, H M. | Krishnaswamy, S. | Lamzin, V. | Manicka, S. | Peleg, Y. | Sussman, J L. | Unger, T. | ISPC, Israel Structural Proteomics Center. | ISPC | Israel Structural Proteomics Center | Dhdp | Dihydrodipicolinic acid synthase | Lyase | N-acetyl neuraminate lyase | Nal | Prophage