Phosphoglycerate Kinase
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
== PGK in the Glycolysis Cycle == | == PGK in the Glycolysis Cycle == | ||
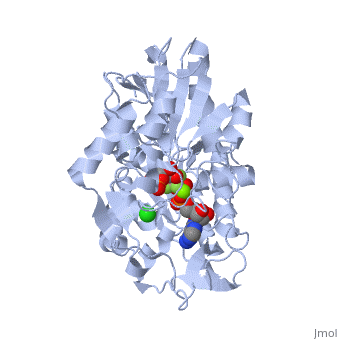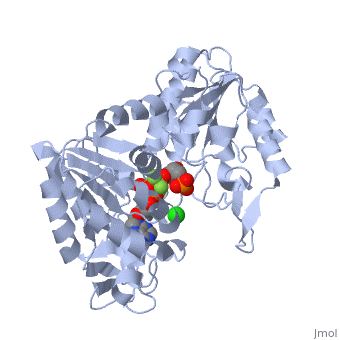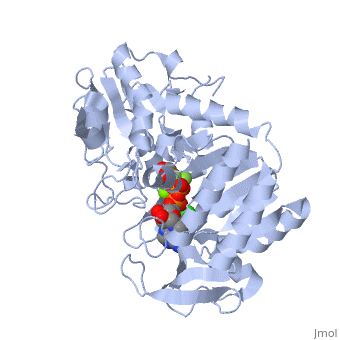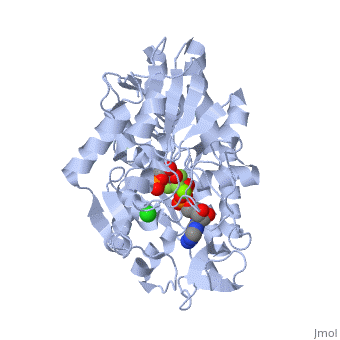
| - | '''Phosphoglycerate kinase''' is a crucial enzyme in the glycolysis cycle. This cycle is a series of ten reactions which ultimately breaks down glucose into pyruvate while generating 2 NADH and 2 ATP molecules. Phosphoglycerate kinase is the seventh enzyme in the cycle which catalyzes the reaction of 1,3-Biphosphoglycerate and ADP to produce <scene name='Shane_Harmon_Sandbox/Product/2'>3-Phosphoglycerate</scene> and <scene name='Shane_Harmon_Sandbox/Atp/4'>ATP</scene>. This method for ATP production is known as substrate-level phosphorylation because it produces energy storing ATP molecules without the use of oxygen, NADH, or an ATPase. The reaction is highly exergonic allowing it to be coupled with the less thermodynamically favored GADPH reaction of the cycle so both reactions occur spontaneously. See [[Glycolysis Enzymes]]. | + | '''Phosphoglycerate kinase''' is a crucial enzyme in the glycolysis cycle. This cycle is a series of ten reactions which ultimately breaks down glucose into pyruvate while generating 2 NADH and 2 ATP molecules. Phosphoglycerate kinase is the seventh enzyme in the cycle which catalyzes the reaction of 1,3-Biphosphoglycerate and ADP to produce <scene name='Shane_Harmon_Sandbox/Product/2'>3-Phosphoglycerate</scene> and <scene name='Shane_Harmon_Sandbox/Atp/4'>ATP</scene>. This method for ATP production is known as substrate-level phosphorylation because it produces energy storing ATP molecules without the use of oxygen, NADH, or an ATPase. The reaction is highly exergonic allowing it to be coupled with the less thermodynamically favored GADPH reaction of the cycle so both reactions occur spontaneously. See [[Glycolysis Enzymes]], [[Gluconeogenesis]]. |
== Structure == | == Structure == | ||
Current revision
| |||||||||||
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- ↑ 1.0 1.1 1.2 1.3 Auerbach G, Huber R, Grattinger M, Zaiss K, Schurig H, Jaenicke R, Jacob U. Closed structure of phosphoglycerate kinase from Thermotoga maritima reveals the catalytic mechanism and determinants of thermal stability. Structure. 1997 Nov 15;5(11):1475-83. PMID:9384563
- ↑ Lallemand P, Chaloin L, Roy B, Barman T, Bowler MW, Lionne C. Interaction of human 3-phosphoglycerate kinase with its two substrates: is substrate antagonism a kinetic advantage? J Mol Biol. 2011 Jun 24;409(5):742-57. Epub 2011 Apr 27. PMID:21549713 doi:10.1016/j.jmb.2011.04.048
- ↑ Voet, Donald et al. 2008. Fundamentals of Biochemistry. 3rd ed. 499
- ↑ Blake CC, Rice DW. Phosphoglycerate kinase. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):93-104. PMID:6115427
- ↑ Vas M, Varga A, Graczer E. Insight into the Mechanism of Domain Movements and their Role in Enzyme Function: Example of 3-Phosphoglycerate Kinase. Curr Protein Pept Sci. 2010 Jan 21. PMID:20088776
- ↑ Haran G, Haas E, Szpikowska BK, Mas MT. Domain motions in phosphoglycerate kinase: determination of interdomain distance distributions by site-specific labeling and time-resolved fluorescence energy transfer. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11764-8. PMID:1465395
- ↑ Scopes RK. The steady-state kinetics of yeast phosphoglycerate kinase. Anomalous kinetic plots and the effects of salts on activity. Eur J Biochem. 1978 Apr 17;85(2):503-16. PMID:348474
- ↑ Macioszek J, Anderson JB, Anderson LE. Isolation of chloroplastic phosphoglycerate kinase : kinetics of the two-enzyme phosphoglycerate kinase/glyceraldehyde-3-phosphate dehydrogenase couple. Plant Physiol. 1990 Sep;94(1):291-6. PMID:16667700
- ↑ Wu S, Storey JM, Storey KB. Phosphoglycerate kinase 1 expression responds to freezing, anoxia, and dehydration stresses in the freeze tolerant wood frog, Rana sylvatica. J Exp Zool A Ecol Genet Physiol. 2009 Jan 1;311(1):57-67. doi: 10.1002/jez.495. PMID:18785212 doi:http://dx.doi.org/10.1002/jez.495
- ↑ Hogg PJ. Biological regulation through protein disulfide bond cleavage. Redox Rep. 2002;7(2):71-7. doi: 10.1179/135100002125000299. PMID:12189052 doi:http://dx.doi.org/10.1179/135100002125000299
Proteopedia Page Contributors and Editors (what is this?)
Shane Harmon, Michal Harel, Joel L. Sussman, Brandon Tritle, David Canner, Alexander Berchansky