Ceramidase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<StructureSection load='2zxc' size='340' side='right' caption="CerN bound to C2 Ceramide [[2zxc]]", [[Resolution|resolution]] 2.20Å' scene='91/910024/2zxc_ballstick/2'> | <StructureSection load='2zxc' size='340' side='right' caption="CerN bound to C2 Ceramide [[2zxc]]", [[Resolution|resolution]] 2.20Å' scene='91/910024/2zxc_ballstick/2'> | ||
| - | == | + | == Introduction == |
| + | '''Ceramidase''' is a key enzyme in degradation of ceramide into sphingosine and free fatty acid. Several ceramidases are known in human: | ||
| + | *Neutral ceramidase <ref>PMID:16380386</ref> | ||
| + | *Acid ceramidase.<ref>PMID:17064658</ref> | ||
| + | *Alkaline ceramidase.<ref>PMID:33271224</ref> | ||
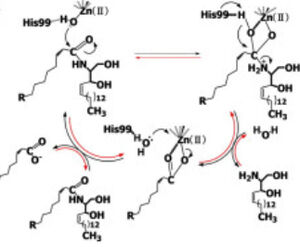
[[Image:CerN Mechanism Okino2009.jpg|300px|right|thumb|'''Figure 1''' Proposed mechanisms of the zinc-dependent hydrolysis of C2-ceramide (Black arrows) and the zinc-dependent synthesis of C2-ceramide from palmitate and sphingosine (Red arrows) by CerN . Figure adapted from Inoe et al.(2009)<ref name="Inoue">PMID:19088069</ref>]] | [[Image:CerN Mechanism Okino2009.jpg|300px|right|thumb|'''Figure 1''' Proposed mechanisms of the zinc-dependent hydrolysis of C2-ceramide (Black arrows) and the zinc-dependent synthesis of C2-ceramide from palmitate and sphingosine (Red arrows) by CerN . Figure adapted from Inoe et al.(2009)<ref name="Inoue">PMID:19088069</ref>]] | ||
| + | == Function == | ||
'''Neutral ceramidase''' '''CerN''' is an enzyme that catalyzes the cleavage of the [https://en.wikipedia.org/wiki/Sphingolipid Sphingolipid] <scene name='91/910024/Ceramide/3'>Ceramide</scene> at the <scene name='91/910024/Ceramide18/1'>N-acyl linkage</scene>, producing <scene name='91/910024/Ceramide/4'>sphingosine and a fatty acid</scene>.<ref name="Okino">PMID:9603946</ref> <ref name="Inoue">PMID:19088069</ref> As a neutral ceramidase, optimal catalytic activity of CerN occurs between pH 6.5-8.5.<ref name="Okino">PMID:9603946</ref> CerN cleaves the N-acyl linkage within ceramides via '''zinc-dependent hydrolysis''' and the enzyme is also capable of '''synthesizing ceramide''' from sphingosine and palmitic acid by the reverse mechanism.<ref name="Inoue">PMID:19088069</ref><ref name="Reverse">PMID:10832092</ref> The zinc ion within the <scene name='91/910024/Activesite2/3'>active site</scene> is coordinated by His97, His204, Glu411, Tyr448, and a water molecule. His97 and Tyr448 are required for zinc binding within the active site. ''Ligand binding within the active site is recognized by Gly25, His99, Arg160, and Tyr460''.<ref name="Inoue">PMID:19088069</ref> Ser27 and Gly25 stabilize ceramide within the active site by forming a water-mediated hydrogen bond with the central OH of ceramide, and the carbonyl oxygen is stabilized by the Tyr448 and Tyr460. | '''Neutral ceramidase''' '''CerN''' is an enzyme that catalyzes the cleavage of the [https://en.wikipedia.org/wiki/Sphingolipid Sphingolipid] <scene name='91/910024/Ceramide/3'>Ceramide</scene> at the <scene name='91/910024/Ceramide18/1'>N-acyl linkage</scene>, producing <scene name='91/910024/Ceramide/4'>sphingosine and a fatty acid</scene>.<ref name="Okino">PMID:9603946</ref> <ref name="Inoue">PMID:19088069</ref> As a neutral ceramidase, optimal catalytic activity of CerN occurs between pH 6.5-8.5.<ref name="Okino">PMID:9603946</ref> CerN cleaves the N-acyl linkage within ceramides via '''zinc-dependent hydrolysis''' and the enzyme is also capable of '''synthesizing ceramide''' from sphingosine and palmitic acid by the reverse mechanism.<ref name="Inoue">PMID:19088069</ref><ref name="Reverse">PMID:10832092</ref> The zinc ion within the <scene name='91/910024/Activesite2/3'>active site</scene> is coordinated by His97, His204, Glu411, Tyr448, and a water molecule. His97 and Tyr448 are required for zinc binding within the active site. ''Ligand binding within the active site is recognized by Gly25, His99, Arg160, and Tyr460''.<ref name="Inoue">PMID:19088069</ref> Ser27 and Gly25 stabilize ceramide within the active site by forming a water-mediated hydrogen bond with the central OH of ceramide, and the carbonyl oxygen is stabilized by the Tyr448 and Tyr460. | ||
Upon ligand binding, CerN enters the <scene name='91/910024/Closedsurf_use/1'>closed</scene> conformation. <ref name="Inoue">PMID:19088069</ref> ''His99 and Arg160 function in the catalysis of ceramide hydrolysis'', as they deprotonate their coordinated water molecule to produce a hydroxide ion.<ref name="Inoue">PMID:19088069</ref> The carbonyl carbon of ceramide undergoes a '''nucleophilic attack''' by the hydroxide ion ('''Figure 1''').<ref name="Inoue">PMID:19088069</ref> The carbonyl oxygen stabilized by Tyr448 and Tyr460 is then passed to the zinc ion, allowing for the breakage of the N-acyl linkage.<ref name="Inoue">PMID:19088069</ref> Sphingosine is then released from the active site while the fatty acid remains bound to the zinc ion until it is replaced by a new water molecule, shifting CerN into the <scene name='91/910024/Activesite_open_surf/2'>open</scene> conformation.<ref name="Inoue">PMID:19088069</ref> The synthesis of ceramide from palmitate and sphingosine occurs via the same mechanism but in reverse ('''Figure 1'''). <ref name="Inoue">PMID:19088069</ref> | Upon ligand binding, CerN enters the <scene name='91/910024/Closedsurf_use/1'>closed</scene> conformation. <ref name="Inoue">PMID:19088069</ref> ''His99 and Arg160 function in the catalysis of ceramide hydrolysis'', as they deprotonate their coordinated water molecule to produce a hydroxide ion.<ref name="Inoue">PMID:19088069</ref> The carbonyl carbon of ceramide undergoes a '''nucleophilic attack''' by the hydroxide ion ('''Figure 1''').<ref name="Inoue">PMID:19088069</ref> The carbonyl oxygen stabilized by Tyr448 and Tyr460 is then passed to the zinc ion, allowing for the breakage of the N-acyl linkage.<ref name="Inoue">PMID:19088069</ref> Sphingosine is then released from the active site while the fatty acid remains bound to the zinc ion until it is replaced by a new water molecule, shifting CerN into the <scene name='91/910024/Activesite_open_surf/2'>open</scene> conformation.<ref name="Inoue">PMID:19088069</ref> The synthesis of ceramide from palmitate and sphingosine occurs via the same mechanism but in reverse ('''Figure 1'''). <ref name="Inoue">PMID:19088069</ref> | ||
Revision as of 08:16, 18 January 2023
| |||||||||||