|
|
| Line 1: |
Line 1: |
| | | | |
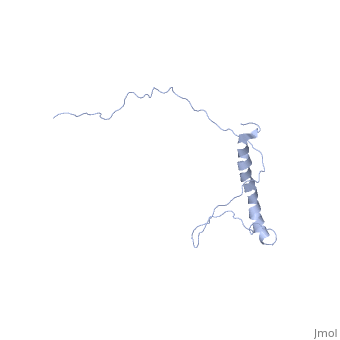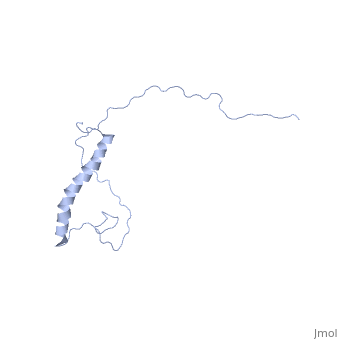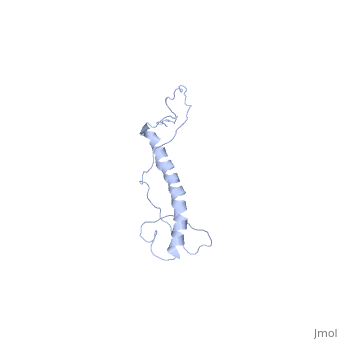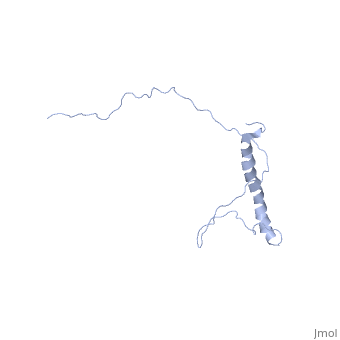
| | ==NMR structure of Hsp12 in the presence of DPC== | | ==NMR structure of Hsp12 in the presence of DPC== |
| - | <StructureSection load='2ljl' size='340' side='right'caption='[[2ljl]], [[NMR_Ensembles_of_Models | 20 NMR models]]' scene=''> | + | <StructureSection load='2ljl' size='340' side='right'caption='[[2ljl]]' scene=''> |
| | == Structural highlights == | | == Structural highlights == |
| - | <table><tr><td colspan='2'>[[2ljl]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Baker's_yeast Baker's yeast]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2LJL OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2LJL FirstGlance]. <br> | + | <table><tr><td colspan='2'>[[2ljl]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Saccharomyces_cerevisiae_S288C Saccharomyces cerevisiae S288C]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2LJL OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2LJL FirstGlance]. <br> |
| - | </td></tr><tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">HSP12, GLP1, HOR5, YFL014W ([https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=559292 Baker's yeast])</td></tr> | + | </td></tr><tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2ljl FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2ljl OCA], [https://pdbe.org/2ljl PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2ljl RCSB], [https://www.ebi.ac.uk/pdbsum/2ljl PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2ljl ProSAT]</span></td></tr> |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2ljl FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2ljl OCA], [https://pdbe.org/2ljl PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2ljl RCSB], [https://www.ebi.ac.uk/pdbsum/2ljl PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2ljl ProSAT]</span></td></tr> | + | |
| | </table> | | </table> |
| | == Function == | | == Function == |
| - | [[https://www.uniprot.org/uniprot/HSP12_YEAST HSP12_YEAST]] May play a role in a switch from carbohydrate utilizing metabolism to fatty acid utilizing metabolism.
| + | [https://www.uniprot.org/uniprot/HSP12_YEAST HSP12_YEAST] May play a role in a switch from carbohydrate utilizing metabolism to fatty acid utilizing metabolism. |
| | <div style="background-color:#fffaf0;"> | | <div style="background-color:#fffaf0;"> |
| | == Publication Abstract from PubMed == | | == Publication Abstract from PubMed == |
| Line 25: |
Line 24: |
| | __TOC__ | | __TOC__ |
| | </StructureSection> | | </StructureSection> |
| - | [[Category: Baker's yeast]] | |
| | [[Category: Large Structures]] | | [[Category: Large Structures]] |
| - | [[Category: Structural genomic]] | + | [[Category: Saccharomyces cerevisiae S288C]] |
| - | [[Category: Markley, J]] | + | [[Category: Markley J]] |
| - | [[Category: Singarapu, K]] | + | [[Category: Singarapu K]] |
| - | [[Category: Cesg]]
| + | |
| - | [[Category: Chaperone]]
| + | |
| - | [[Category: PSI, Protein structure initiative]]
| + | |
| Structural highlights
Function
HSP12_YEAST May play a role in a switch from carbohydrate utilizing metabolism to fatty acid utilizing metabolism.
Publication Abstract from PubMed
Hsp12 (heat shock protein 12) belongs to the small heat shock protein family, partially characterized as a stress response, stationary phase entry, late embryonic abundant-like protein located at the plasma membrane to protect membrane from desiccation. Here, we report the structural characterization of Hsp12 by NMR and biophysical techniques. The protein was labeled uniformly with nitrogen-15 and carbon-13 so that its conformation could be determined in detail both in aqueous solution and in two membrane-mimetic environments, SDS and dodecylphosphocholine (DPC) micelles. Secondary structural elements determined from assigned chemical shifts indicated that Hsp12 is dynamically disordered in aqueous solution, whereas it gains four helical stretches in the presence of SDS micelles and a single helix in presence of DPC. These conclusions were reinforced by circular dichroism spectra of the protein in all three environments. The lack of long range interactions in NOESY spectra indicated that the helices present in SDS micelles do not pack together. R(1) and R(2), relaxation and heteronuclear NOE measurements showed that the protein is disordered in aqueous solution but becomes more ordered in presence of detergent micelles. NMR spectra collected in presence of paramagnetic spin relaxation agents (5DSA, 16DSA, and Gd(DTPA-BMA)) indicated that the amphipathic alpha-helices of Hsp12 in SDS micelles lie on the membrane surface. These observations are in agreement with studies suggesting that Hsp12 functions to protect the membrane from desiccation.
Structural characterization of Hsp12, the heat shock protein from Saccharomyces cerevisiae, in aqueous solution where it is intrinsically disordered and in detergent micelles where it is locally alpha-helical.,Singarapu KK, Tonelli M, Chow DC, Frederick RO, Westler WM, Markley JL J Biol Chem. 2011 Dec 16;286(50):43447-53. Epub 2011 Oct 13. PMID:21998307[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Singarapu KK, Tonelli M, Chow DC, Frederick RO, Westler WM, Markley JL. Structural characterization of Hsp12, the heat shock protein from Saccharomyces cerevisiae, in aqueous solution where it is intrinsically disordered and in detergent micelles where it is locally alpha-helical. J Biol Chem. 2011 Dec 16;286(50):43447-53. Epub 2011 Oct 13. PMID:21998307 doi:http://dx.doi.org/10.1074/jbc.M111.306464
|