We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1782
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
*The bile salts transported by NTCP are located within the gastrointestinal tract of the body and play a very key role in many biological functions. These functions include digesting and absorbing nutrients by helping break down fats and transporting lipid soluble nutrients into the liver. <Ref> Maldonado-Valderrama, J., Wilde, P., Macierzanka, A., & Mackie, A. (2011). The role of bile salts in digestion. Advances in colloid and interface science, 165(1), 36–46. [https://doi.org/10.1016/j.cis.2010.12.002 DOI: 10.1016/j.cis.2010.12.002]. </Ref>. | *The bile salts transported by NTCP are located within the gastrointestinal tract of the body and play a very key role in many biological functions. These functions include digesting and absorbing nutrients by helping break down fats and transporting lipid soluble nutrients into the liver. <Ref> Maldonado-Valderrama, J., Wilde, P., Macierzanka, A., & Mackie, A. (2011). The role of bile salts in digestion. Advances in colloid and interface science, 165(1), 36–46. [https://doi.org/10.1016/j.cis.2010.12.002 DOI: 10.1016/j.cis.2010.12.002]. </Ref>. | ||
| - | *The NTCP carrier protein itself can be found within hepatocytes, or the epithelial cells of the liver, but more specifically, within the basolateral membrane of these cells. <Ref> Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. [https://dx.doi.org/10.1038/s41586-022-04845-4 DOI: 10.1038/s41586-022-04845-4]. </Ref>. The uptake of bile salts into the liver also allow for drugs to be both absorbed and excreted, as well as essential nutrients such as Vitamin A,D,E, and K to be absorbed in the small intestine. | + | *The NTCP carrier protein itself can be found within hepatocytes, or the epithelial cells of the liver, but more specifically, within the basolateral membrane of these cells. <Ref name="Asami"> Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. [https://dx.doi.org/10.1038/s41586-022-04845-4 DOI: 10.1038/s41586-022-04845-4]. </Ref>. The uptake of bile salts into the liver also allow for drugs to be both absorbed and excreted, as well as essential nutrients such as Vitamin A,D,E, and K to be absorbed in the small intestine. |
=== Structural Overview === | === Structural Overview === | ||
| - | + | Tatiana | |
== Function == | == Function == | ||
| - | + | Olivia | |
=== Binding Pocket === | === Binding Pocket === | ||
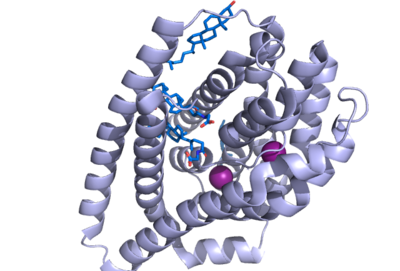
| - | *The NTCP binding pocket represents a "tunnel" lined with hydrophilic residues within the NTCP structure to allow hydrophilic bile salts and sodium ions to bind. When there are no bile salts bound to NTCP's binding tunnel, it forms a hollow hole in the middle of the structure, from <scene name='95/952710/Tunnel_front/1'>front</scene> to <scene name='95/952710/Tunnel_front/3'>back</scene>. As bile salts bind to this binding pocket, the bile salts very nicely fill the hole within NTCP and fit almost perfectly within the tunnel, to the point where the <scene name='95/952710/Bile_bound_to_tunnel/2'>tunnel</scene> can no longer be seen, as it is completely encompassing the bile salts. This tunnel formed connects the external cytoplasm of the hepatocyte to the inner leaflet of the basolateral membrane. The side of the tunnel where the bile salts bind are lined with hydrophilic residues, whereas the opposite side of the helix is lined with hydrophobic residues, as the transmembrane helices are amphipathic. <Ref> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). [https://doi.org/10.1038/s41422-022-00680-4 DOI: 10.1038/s41422-022-00680-4]. </Ref>. | + | *The NTCP binding pocket represents a "tunnel" lined with hydrophilic residues within the NTCP structure to allow hydrophilic bile salts and sodium ions to bind. When there are no bile salts bound to NTCP's binding tunnel, it forms a hollow hole in the middle of the structure, from <scene name='95/952710/Tunnel_front/1'>front</scene> to <scene name='95/952710/Tunnel_front/3'>back</scene>. As bile salts bind to this binding pocket, the bile salts very nicely fill the hole within NTCP and fit almost perfectly within the tunnel, to the point where the <scene name='95/952710/Bile_bound_to_tunnel/2'>tunnel</scene> can no longer be seen, as it is completely encompassing the bile salts. This tunnel formed connects the external cytoplasm of the hepatocyte to the inner leaflet of the basolateral membrane. The side of the tunnel where the bile salts bind are lined with hydrophilic residues, whereas the opposite side of the helix is lined with hydrophobic residues, as the transmembrane helices are amphipathic. <Ref name="Liu"> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). [https://doi.org/10.1038/s41422-022-00680-4 DOI: 10.1038/s41422-022-00680-4]. </Ref>. |
| + | |||
| + | *The binding pocket of NTCP contains two patches on its exterior. These two patches are used for assisting in the binding of bile salts into the binding tunnel. These external patches are extremely important for aiding in the transport of bile salts from the exterior of NTCP to the interior binding tunnel <ref name="Asami"/>. | ||
| + | |||
== Mechanism == | == Mechanism == | ||
| - | + | Olivia | |
== Significance == | == Significance == | ||
| - | + | Olivia | |
=== HBV/ HDV === | === HBV/ HDV === | ||
| - | + | Tatiana | |
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
Revision as of 20:48, 20 March 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
human Sodium Taurocholate Co-transporting Polypeptide (NTCP) structure
| |||||||||||
References
- ↑ Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ Maldonado-Valderrama, J., Wilde, P., Macierzanka, A., & Mackie, A. (2011). The role of bile salts in digestion. Advances in colloid and interface science, 165(1), 36–46. DOI: 10.1016/j.cis.2010.12.002.
- ↑ 3.0 3.1 Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. DOI: 10.1038/s41586-022-04845-4.
- ↑ Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). DOI: 10.1038/s41422-022-00680-4.
Student Contributors
- Kenna King
- Tatiana Pereda
- Olivia Simcox