Sandbox Reserved 1780
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load= <StructureSection load='Edited_7utz.pse' size='340' side='right' caption='Thyroid stimulating hormone receptor bound to thyroid stimulating hormone' scene='95/952708/Tshr_general_structure/3'> | <StructureSection load= <StructureSection load='Edited_7utz.pse' size='340' side='right' caption='Thyroid stimulating hormone receptor bound to thyroid stimulating hormone' scene='95/952708/Tshr_general_structure/3'> | ||
| - | == Active and Inactive Form == | ||
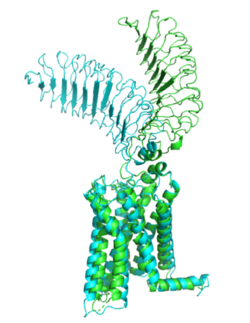
[[Image:Morph_pics2.png|250 px|right|thumb|Figure 1: Inactive form of the thyrotropin receptor shown in blue. Active form of the thyrotropin receptor shown in green.]] | [[Image:Morph_pics2.png|250 px|right|thumb|Figure 1: Inactive form of the thyrotropin receptor shown in blue. Active form of the thyrotropin receptor shown in green.]] | ||
| - | The TSHR protein exists in two states, active and inactive. The <scene name='95/952708/Tshr_chainr_ecd/1'>extracellular domain (ECD)</scene> sticks out from the cell membrane into the space outside the cell. The <scene name='95/952708/Tshr_chainr_tm/1'>transmembrane domain</scene> contains 7 alpha helices that reside within the cell membrane. The <scene name='95/952708/Tshr_chainr/4'>TSHR active form</scene> exists when bound to the thyroid stimulating hormone (TSH) (GREEN LINK). One proposed mechanism for the transition from the active to inactive describes that in its natural state, the TSHR ECD can spontaneously transition to the up state, leading to constitutive activity. In this active state, TSH will bind and keep the active state in the up position because of clash with the cell membrane.<ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> Conformational change of ECD allows for signal transduction through the TM and into the cell. The ECD rotates 55 degrees up in the active form. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> | + | |
| + | |||
| + | == Active and Inactive Form == | ||
| + | |||
| + | The TSHR protein exists in two states, active and inactive. The <scene name='95/952708/Tshr_chainr_ecd/1'>extracellular domain (ECD)</scene> sticks out from the cell membrane into the space outside the cell. The <scene name='95/952708/Tshr_chainr_tm/1'>transmembrane domain</scene> contains 7 alpha helices that reside within the cell membrane. The <scene name='95/952708/Tshr_chainr/4'>TSHR active form</scene> exists when bound to the thyroid stimulating hormone (TSH) (GREEN LINK). One proposed mechanism for the transition from the active to inactive describes that in its natural state, the TSHR ECD can spontaneously transition to the up state, leading to constitutive activity. In this active state, TSH will bind and keep the active state in the up position because of clash with the cell membrane.<ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> Conformational change of ECD allows for signal transduction through the TM and into the cell. The ECD rotates 55 degrees up in the active form. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> | ||
| + | |||
== TSHR Agonists and Antagonists == | == TSHR Agonists and Antagonists == | ||
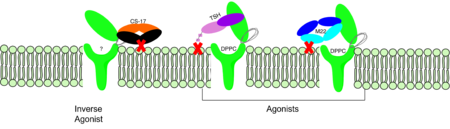
The natural thyroid stimulating hormone binds the ECD and keeps the TSHR in the active state due to steric clash with the cell membrane. This steric clash comes from the ASN 52 (GREEN LINK) residue on the TSH (GREEN LINK). | The natural thyroid stimulating hormone binds the ECD and keeps the TSHR in the active state due to steric clash with the cell membrane. This steric clash comes from the ASN 52 (GREEN LINK) residue on the TSH (GREEN LINK). | ||
| + | |||
===M22 Agonist=== | ===M22 Agonist=== | ||
M22 is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that was isolated from a patient with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. This antibody activates TSHR by causing a membrane clash with the ECD and cell membrane, therefore keeping the TSHR in the activate state by preventing the TSHR from rotating to the inactive state (Figure 2). This autoantibody mimics TSH action and binding to TSHR resulting in a potent activator for TSHR. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> | M22 is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that was isolated from a patient with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. This antibody activates TSHR by causing a membrane clash with the ECD and cell membrane, therefore keeping the TSHR in the activate state by preventing the TSHR from rotating to the inactive state (Figure 2). This autoantibody mimics TSH action and binding to TSHR resulting in a potent activator for TSHR. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> | ||
Revision as of 20:05, 27 March 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1