Sandbox Reserved 1779
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
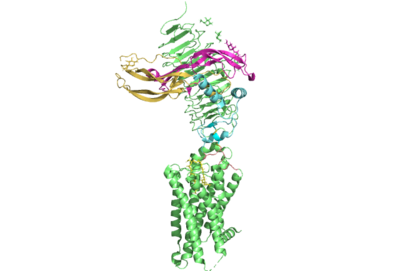
The thyrotropin receptor has an extracellular domain (ECD) that is composed of a <scene name='95/952709/Lrrd_real/2'>leucine rich repeat domain (LRRD)</scene> as well as a hinge region. This <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> links the ECD to the seven transmembrane helices <scene name='95/952709/7tm_helices/4'>(7TM domain)</scene>, which span from the extracellular domain to the intracellular domain <ref name= "Keinau et al.">PMID:228484426</ref>. When thyrotropin or an autoantibody binds, it causes a conformational change in the receptor through the transmembrane helices. This causes the thyrotropin receptor to interact differently with its respective <scene name='95/952709/G_protein/2'>G-protein</scene> when in the active and inactive states. | The thyrotropin receptor has an extracellular domain (ECD) that is composed of a <scene name='95/952709/Lrrd_real/2'>leucine rich repeat domain (LRRD)</scene> as well as a hinge region. This <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> links the ECD to the seven transmembrane helices <scene name='95/952709/7tm_helices/4'>(7TM domain)</scene>, which span from the extracellular domain to the intracellular domain <ref name= "Keinau et al.">PMID:228484426</ref>. When thyrotropin or an autoantibody binds, it causes a conformational change in the receptor through the transmembrane helices. This causes the thyrotropin receptor to interact differently with its respective <scene name='95/952709/G_protein/2'>G-protein</scene> when in the active and inactive states. | ||
=== Leucine Rich Region === | === Leucine Rich Region === | ||
| - | The Leucine Rich region is part of the <scene name='95/952708/Tshr_chainr_ecd/1'>extracellular domain (ECD)</scene> of TSHR. The highlighted region contains <scene name='95/952707/Lrr/3'>10-11 Leucine Repeats</scene> within the structure. The specific residues from TSHR interacting with TSH are <scene name='95/952707/Lrr/2'>Lys209 and Lys 58</scene> <ref name="Duan et al.">PMID: 35940204</ref>. These interact with Asp91 and Glu98 in the seatbelt region of TSH forming a salt bridge and initiating the conformational change in the receptor <ref name="Faust | + | The Leucine Rich region is part of the <scene name='95/952708/Tshr_chainr_ecd/1'>extracellular domain (ECD)</scene> of TSHR. The highlighted region contains <scene name='95/952707/Lrr/3'>10-11 Leucine Repeats</scene> within the structure. The specific residues from TSHR interacting with TSH are <scene name='95/952707/Lrr/2'>Lys209 and Lys 58</scene> <ref name="Duan et al.">PMID: 35940204</ref>. These interact with Asp91 and Glu98 in the seatbelt region of TSH forming a salt bridge and initiating the conformational change in the receptor <ref name="Faust">PMID: 35940205</ref>. This interaction is specific to TSH and TSHR. When other agonists or antagonists bind to the receptor, the interaction is a result of different residues interacting. The Leucine residues likely play a role in how the ECD folds and which residues are located on the exterior protein. As Leucine is hydrophobic, it would be forced into the interior of the protein during folding exposing other residues that are more hydrophobic to the surface. |
=== Active and Inactive Form === | === Active and Inactive Form === | ||
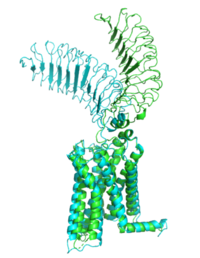
[[Image:Morph_pics2.png|200 px|right|thumb|Figure 1: Inactive form of the thyrotropin receptor shown in blue. Active form of the thyrotropin receptor shown in green.]] | [[Image:Morph_pics2.png|200 px|right|thumb|Figure 1: Inactive form of the thyrotropin receptor shown in blue. Active form of the thyrotropin receptor shown in green.]] | ||
| - | The TSHR protein exists in two states, active and inactive (Figure 1). The <scene name='95/952708/Tshr_chainr_ecd/1'>extracellular domain (ECD)</scene> sticks out from the cell membrane into the space outside the cell. The <scene name='95/952708/Tshr_chainr_tm/1'>transmembrane domain</scene> contains 7 alpha helices that reside within the cell membrane. The <scene name='95/952708/Tshr_chainr/4'>TSHR active form</scene> exists when bound to the thyroid stimulating hormone (TSH) (GREEN LINK). One proposed mechanism for the transition from the active to inactive describes that in its natural state, the TSHR ECD can spontaneously transition to the up state, leading to constitutive activity. In this active state, TSH will bind and keep the active state in the up position because of clash with the cell membrane.<ref name="Faust" | + | The TSHR protein exists in two states, active and inactive (Figure 1). The <scene name='95/952708/Tshr_chainr_ecd/1'>extracellular domain (ECD)</scene> sticks out from the cell membrane into the space outside the cell. The <scene name='95/952708/Tshr_chainr_tm/1'>transmembrane domain</scene> contains 7 alpha helices that reside within the cell membrane. The <scene name='95/952708/Tshr_chainr/4'>TSHR active form</scene> exists when bound to the thyroid stimulating hormone (TSH) (GREEN LINK). One proposed mechanism for the transition from the active to inactive describes that in its natural state, the TSHR ECD can spontaneously transition to the up state, leading to constitutive activity. In this active state, TSH will bind and keep the active state in the up position because of clash with the cell membrane.<ref name="Faust" /> Conformational change of ECD allows for signal transduction through the TM and into the cell. The ECD rotates 55 degrees up in the active form. <ref name="Faust" /> |
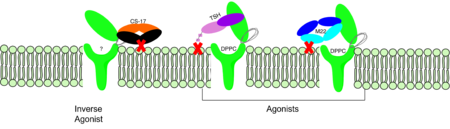
== TSHR Agonists and Antagonists == | == TSHR Agonists and Antagonists == | ||
Revision as of 00:10, 29 March 2023
>
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.
- ↑ 2.0 2.1 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.
- ↑ . PMID:228484426
- ↑ 5.0 5.1 5.2 5.3 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ Nunez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009 May;42(5):381-95. Epub 2009 Feb 16. PMID:19221175 doi:10.1677/JME-08-0152