We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1779
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
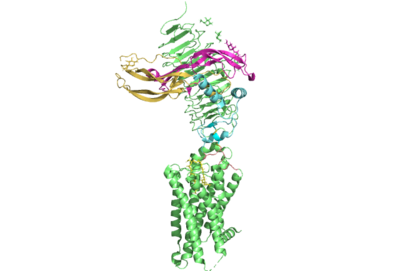
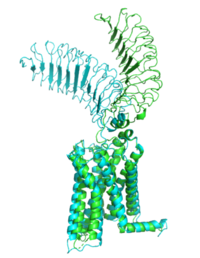
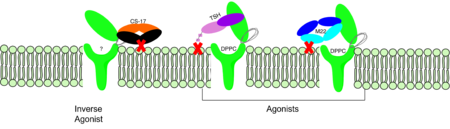
The thyrotropin receptor has an extracellular domain (ECD) that is composed of a <scene name='95/952709/Lrrd_real/2'>leucine rich repeat domain (LRRD)</scene> as well as a hinge region. This <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> links the ECD to the seven transmembrane helices <scene name='95/952709/7tm_helices/4'>(7TM domain)</scene>, which span from the extracellular domain to the intracellular domain <ref name= "Keinau et al.">PMID:228484426</ref>. When thyrotropin or an autoantibody binds, it causes a conformational change in the receptor through the transmembrane helices. This causes the thyrotropin receptor to interact differently with its respective <scene name='95/952709/G_protein/2'>G-protein</scene> when in the active and inactive states. | The thyrotropin receptor has an extracellular domain (ECD) that is composed of a <scene name='95/952709/Lrrd_real/2'>leucine rich repeat domain (LRRD)</scene> as well as a hinge region. This <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> links the ECD to the seven transmembrane helices <scene name='95/952709/7tm_helices/4'>(7TM domain)</scene>, which span from the extracellular domain to the intracellular domain <ref name= "Keinau et al.">PMID:228484426</ref>. When thyrotropin or an autoantibody binds, it causes a conformational change in the receptor through the transmembrane helices. This causes the thyrotropin receptor to interact differently with its respective <scene name='95/952709/G_protein/2'>G-protein</scene> when in the active and inactive states. | ||
=== Leucine Rich Region === | === Leucine Rich Region === | ||
| - | The Leucine Rich region is part of the <scene name='95/952708/Tshr_chainr_ecd/1'>extracellular domain (ECD)</scene> of TSHR. The highlighted region contains <scene name='95/952707/Lrr/3'>10-11 Leucine Repeats</scene> within the structure. The specific residues from TSHR interacting with TSH are <scene name='95/952707/Lrr/2'>Lys209 and | + | The Leucine Rich region is part of the <scene name='95/952708/Tshr_chainr_ecd/1'>extracellular domain (ECD)</scene> of TSHR. The highlighted region contains <scene name='95/952707/Lrr/3'>10-11 Leucine Repeats</scene> within the structure. The specific residues from TSHR interacting with TSH are <scene name='95/952707/Lrr/2'>Lys209 and Lys58</scene> <ref name="Duan et al.">PMID: 35940204</ref>. These interact with <scene name='95/952709/Interactions_with_thyrotropin/1'>Glu 98 and Asp 91</scene> in the seatbelt region of TSH forming a salt bridge and initiating the conformational change in the receptor <ref name="Faust">PMID: 35940205</ref>. This interaction is specific to TSH and TSHR. When other agonists or antagonists bind to the receptor, the interaction is a result of different residues interacting. The Leucine residues likely play a role in how the ECD folds and which residues are located on the exterior protein. As Leucine is hydrophobic, it would be forced into the interior of the protein during folding exposing other residues that are more hydrophobic to the surface. |
=== Active and Inactive Form === | === Active and Inactive Form === | ||
Revision as of 00:39, 29 March 2023
>
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.
- ↑ 2.0 2.1 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.
- ↑ . PMID:228484426
- ↑ 5.0 5.1 5.2 5.3 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ Nunez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009 May;42(5):381-95. Epub 2009 Feb 16. PMID:19221175 doi:10.1677/JME-08-0152