We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1780
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
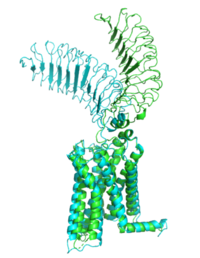
The TSHR protein exists in two states, active and inactive (Figure 1). The extracellular domain <scene name='95/952708/Ecd/4'>(ECD)</scene> sticks out from the cell membrane into the space outside the cell. The <scene name='95/952709/7tm_helices/4'>7TM domain</scene> contains 7 alpha helices that reside within the cell membrane. The <scene name='95/952708/Tshr_chainr/4'>TSHR active form</scene> exists when bound to the thyroid stimulating hormone (TSH) (GREEN LINK). One proposed mechanism for the transition from the active to inactive describes that in its natural state, the TSHR ECD can spontaneously transition to the up state, leading to constitutive activity. In this active state, TSH will bind and keep the active state in the up position because of clash with the cell membrane.<ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> Conformational change of ECD allows for signal transduction through the TM and into the cell. The ECD rotates 55 degrees up in the active form. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> | The TSHR protein exists in two states, active and inactive (Figure 1). The extracellular domain <scene name='95/952708/Ecd/4'>(ECD)</scene> sticks out from the cell membrane into the space outside the cell. The <scene name='95/952709/7tm_helices/4'>7TM domain</scene> contains 7 alpha helices that reside within the cell membrane. The <scene name='95/952708/Tshr_chainr/4'>TSHR active form</scene> exists when bound to the thyroid stimulating hormone (TSH) (GREEN LINK). One proposed mechanism for the transition from the active to inactive describes that in its natural state, the TSHR ECD can spontaneously transition to the up state, leading to constitutive activity. In this active state, TSH will bind and keep the active state in the up position because of clash with the cell membrane.<ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> Conformational change of ECD allows for signal transduction through the TM and into the cell. The ECD rotates 55 degrees up in the active form. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> | ||
| - | == TSHR Agonists and | + | == TSHR Agonists and Inverse Agonists == |
| - | Chemical [https://en.wikipedia.org/wiki/Agonist agonists] are found in many living systems and serve as a way to activate receptors or pathways that are necessary for a wide array of biological processes. Chemical [https://en.wikipedia.org/wiki/ | + | Chemical [https://en.wikipedia.org/wiki/Agonist agonists] are found in many living systems and serve as a way to activate receptors or pathways that are necessary for a wide array of biological processes. Chemical [https://en.wikipedia.org/wiki/Inverse_agonist inverse agonists] bind to the same receptor as the agonist but promotes a biological response opposite that of the agonist. Different types of agonists exist within the body including hormones, antibodies, and neurotransmitters. The body naturally produces autoantibodies that can act as agonists and mimic the activating mechanism of the natural hormone. Isolating these antibodies in patients with diseases can lead researches to uncover the mechanism of binding for the receptor. |
===M22 Agonist=== | ===M22 Agonist=== | ||
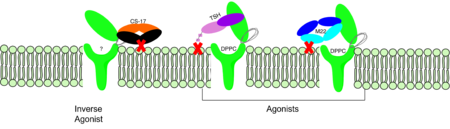
| - | M22 is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that was isolated from a patient with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. In Graves' disease, TSHR autoantibodies like M22 mimic TSH function and cause thyroid overactivity. <ref name="Miguel"> doi:10.1677/JME-08-0152</ref>. The M22 [https://en.wikipedia.org/wiki/Autoantibody autoantibody] activates TSHR by causing a membrane clash with the ECD and cell membrane, therefore keeping the TSHR in the activate state by preventing the TSHR from rotating to the inactive state (Figure 2). This autoantibody mimics TSH action and binding to TSHR resulting in a potent activator for TSHR. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> Although M22 binds in a similar manner to TSH, there is a key difference in binding between the two that can reveal the function of the hinge region (GREEN LINK). M22 does not make interactions with the hinge region when bound to TSHR, whereas TSH bound to TSHR does.<ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> This finding shows that the hinge region is not necessary for activation of TSHR, and leads to the discovery other methods of activation. | + | M22 is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that was isolated from a patient with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. In Graves' disease, TSHR autoantibodies like M22 mimic TSH function and cause thyroid overactivity. <ref name="Miguel"> doi:10.1677/JME-08-0152</ref>. The M22 [https://en.wikipedia.org/wiki/Autoantibody autoantibody] activates TSHR by causing a membrane clash with the ECD and cell membrane, therefore keeping the TSHR in the activate state by preventing the TSHR from rotating to the inactive state (Figure 2). This autoantibody mimics TSH action and binding to TSHR resulting in a potent activator for TSHR. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> Although M22 binds in a similar manner to TSH, there is a key difference in binding between the two that can reveal the function of the hinge region (GREEN LINK). M22 does not make interactions with the hinge region when bound to TSHR, whereas TSH bound to TSHR does.<ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> This finding shows that the hinge region is not necessary for activation of TSHR, and leads to the discovery other methods of activation. |
===CS-17 Inverse Agonist=== | ===CS-17 Inverse Agonist=== | ||
| + | |||
[[Image:Agonist pic.png|450 px|right|thumb|Figure 2: Agonist and antagonist drugs for activating or inactivating the TSHR protein.]] | [[Image:Agonist pic.png|450 px|right|thumb|Figure 2: Agonist and antagonist drugs for activating or inactivating the TSHR protein.]] | ||
Revision as of 20:52, 29 March 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ Nunez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009 May;42(5):381-95. Epub 2009 Feb 16. PMID:19221175 doi:10.1677/JME-08-0152