Sandbox Reserved 1771
From Proteopedia
| Line 5: | Line 5: | ||
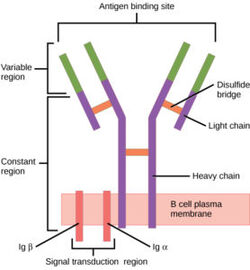
The [https://en.wikipedia.org/wiki/B-cell_receptor human B-cell receptor](BCR) is a complex protein made up of three domains: extracellular, transmembrane, and intracellular. While the extracellular region makes up most of the protein, perhaps the most interesting interactions can be found in the transmembrane domain. Unlike other BCRs, the IgM BCR has a specific heavy chain interaction with the α-β subunit of the protein. BCRs are found on the surface of B-cells as membrane bound proteins (ref). In general the role of BCRs is to bind to foreign antigens and initiate the appropriate immune response. | The [https://en.wikipedia.org/wiki/B-cell_receptor human B-cell receptor](BCR) is a complex protein made up of three domains: extracellular, transmembrane, and intracellular. While the extracellular region makes up most of the protein, perhaps the most interesting interactions can be found in the transmembrane domain. Unlike other BCRs, the IgM BCR has a specific heavy chain interaction with the α-β subunit of the protein. BCRs are found on the surface of B-cells as membrane bound proteins (ref). In general the role of BCRs is to bind to foreign antigens and initiate the appropriate immune response. | ||
==Structure== | ==Structure== | ||
| + | The BCR consists of a membrane-bound immunoglobulin (mIg) and two signal-transducing subunits, Igα and Igβ. The mIg consists of two heavy chains and two light chains. Antigen binding at the binding site triggers signal transduction by Igα and Igβ. Both consist of an extracellular, Ig-like domain, and an intracellular immunoreceptor tyrosine-based activation motif. <Ref name="Su Q"> Su Q, Chen M, Shi Y, Zhang X, Huang G, Huang B, Liu D, Liu Z, Shi Y. Cryo-EM structure of the human IgM B cell receptor. Science. 2022 Aug 19;377(6608):875-880. [doi: 10.1126/science.abo3923. Epub 2022 Aug 18. PMID: 35981043.]</Ref>. | ||
<StructureSection load='4INS' size='350' side='right' caption='Human IgM B-Cell Receptor, 7XQ8 (edited)' scene='95/952699/Overview_rock/1'> | <StructureSection load='4INS' size='350' side='right' caption='Human IgM B-Cell Receptor, 7XQ8 (edited)' scene='95/952699/Overview_rock/1'> | ||
| Line 12: | Line 13: | ||
===Fc and α/β Interactions=== | ===Fc and α/β Interactions=== | ||
| - | + | While the antigen binding site structure of the mIgM BCR is identical to other common soluble antibodies, there are several intracellular interactions between the heavy chains and Igα/β subunits that make it unique. In the Fc portion of the structure, the two heavy chains interact via a disulfide bond and form an <scene name='95/952700/O-shaped_ring/3'>O-shaped ring</scene>. Additionally, the Fc portion binds the <scene name='95/952700/O-shaped_ring/2'>Ig α/β heterodimer</scene> with 1:1 stoichiometry. <Ref name="Tolar P"> Tolar P, Pierce SK. Unveiling the B cell receptor structure. Science. 2022 Aug 19;377(6608):819-820. [doi: 10.1126/science.add8065. Epub 2022 Aug 18. PMID: 35981020.] </Ref>. Due to the orientation of the heavy chains in the O-shaped ring, only Heavy chain 1 (Hc1) forms direct interactions with the Igα/β heterodimer. Furthermore, <scene name='95/952700/Ig-a_and_hc_1/3'>Hc1 and Igα interact</scene> through two hydrogen bonds (T75-Q487 and N73-Q493) which are stabilized by sandwiching of aromatic residues (W76 sandwiched between F358 and F485). Similarly, <scene name='95/952700/Igb_and_hc/1'>Hc1 and Igβ interact</scene> through three hydrogen bonds (Y66-R491, K62-T530, and R55-T533,). The residues involved in the interactions at the heavy chain and Igα/β interface are highly conserved across all species, suggesting a conserved mode of interaction. <Ref name="Su Q"> Su Q, Chen M, Shi Y, Zhang X, Huang G, Huang B, Liu D, Liu Z, Shi Y. Cryo-EM structure of the human IgM B cell receptor. Science. 2022 Aug 19;377(6608):875-880. [doi: 10.1126/science.abo3923. Epub 2022 Aug 18. PMID: 35981043.]</Ref>. The Igα/β heterodimer is an obligate component of all BCRs. Igα and Igβ non-covalently associate with mIgM, and are crucial components for initiating biochemical signaling inside the B cell upon antigen binding. <Ref name="Tolar P"> Tolar P, Pierce SK. Unveiling the B cell receptor structure. Science. 2022 Aug 19;377(6608):819-820. [doi: 10.1126/science.add8065. Epub 2022 Aug 18. PMID: 35981020.] </Ref>. <scene name='95/952700/Iga_and_igb/1'>Igα and Igβ are associated</scene> by a disulfide bond between cystine residues (C119-C136). The disulfide bond is stabilized by π-π stacking (Y122 and F52) and a hydrogen bond (G120-R51). These residues are highly conserved across species, suggesting conservation of the Igα/β interface. | |
===Transmembrane Interactions=== | ===Transmembrane Interactions=== | ||
Many transmembrane interactions can be found within a IgM B-cell receptor. The <scene name='95/952699/Transmembrane_region/1'>α and β subunits</scene> have numerous interactions that keep them associated with each other. An overview of all residue interactions can be found <scene name='95/952699/Overview_hbonds_fixed/1'>here</scene> (highlighted in green). At a cellular pH, various amino acid residues found in the transmembrane region are charged as well, which strengthens the overall interaction. For example, <scene name='95/952699/N155_e138_hbonds_fixed/1'>a hydrogen bond</scene> between residues N155 and E138, along with numerous other hydrogen bonds, works to stabilize the α-β chain interactions. Further down the chains, <scene name='95/952699/T166_e148_hbonds_fixed/1'>interactions</scene> between residues T166 and E148 also have strong hydrogen bonding. Overall, these hydrogen bonds and ion interactions work to maintain the association of the α-β chains. | Many transmembrane interactions can be found within a IgM B-cell receptor. The <scene name='95/952699/Transmembrane_region/1'>α and β subunits</scene> have numerous interactions that keep them associated with each other. An overview of all residue interactions can be found <scene name='95/952699/Overview_hbonds_fixed/1'>here</scene> (highlighted in green). At a cellular pH, various amino acid residues found in the transmembrane region are charged as well, which strengthens the overall interaction. For example, <scene name='95/952699/N155_e138_hbonds_fixed/1'>a hydrogen bond</scene> between residues N155 and E138, along with numerous other hydrogen bonds, works to stabilize the α-β chain interactions. Further down the chains, <scene name='95/952699/T166_e148_hbonds_fixed/1'>interactions</scene> between residues T166 and E148 also have strong hydrogen bonding. Overall, these hydrogen bonds and ion interactions work to maintain the association of the α-β chains. | ||
| - | |||
| - | |||
</StructureSection> | </StructureSection> | ||
Revision as of 21:14, 29 March 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Contents |
IgM B-cell Receptor
Introduction
The human B-cell receptor(BCR) is a complex protein made up of three domains: extracellular, transmembrane, and intracellular. While the extracellular region makes up most of the protein, perhaps the most interesting interactions can be found in the transmembrane domain. Unlike other BCRs, the IgM BCR has a specific heavy chain interaction with the α-β subunit of the protein. BCRs are found on the surface of B-cells as membrane bound proteins (ref). In general the role of BCRs is to bind to foreign antigens and initiate the appropriate immune response.
Structure
The BCR consists of a membrane-bound immunoglobulin (mIg) and two signal-transducing subunits, Igα and Igβ. The mIg consists of two heavy chains and two light chains. Antigen binding at the binding site triggers signal transduction by Igα and Igβ. Both consist of an extracellular, Ig-like domain, and an intracellular immunoreceptor tyrosine-based activation motif. [1].
| |||||||||||
Function
Once bound to an antigen, BCRs undergo a conformational change in the extracellular region. While the exact conformational change is still not known, it initiates several signal transduction pathways. These pathways are responsible for processing the antigen and initiating the appropriate immune responses. More specifically, the α-β subunit is connected to the phosphorylation of an immunoreceptor tyrosine-based activation motif(ITAM) upon binding. This in turn triggers the activation of kinases downstream that aid in the immune response. BCRs can be oligomeric prior to antigen binding, but once bound become an active monomer.
Medical Relevancy
B-cell Formation
The formation of B-cells occurs in the bone marrow. BCRs are attached to B-cells through the aid of membrane-bound proteins in bone marrow cells. During this process, gene recombination occurs, which allows unique BCRs to become highly specific to different antigens.
Diseases
Therapeutics
References
- ↑ 1.0 1.1 Su Q, Chen M, Shi Y, Zhang X, Huang G, Huang B, Liu D, Liu Z, Shi Y. Cryo-EM structure of the human IgM B cell receptor. Science. 2022 Aug 19;377(6608):875-880. [doi: 10.1126/science.abo3923. Epub 2022 Aug 18. PMID: 35981043.]
- ↑ 2.0 2.1 Tolar P, Pierce SK. Unveiling the B cell receptor structure. Science. 2022 Aug 19;377(6608):819-820. [doi: 10.1126/science.add8065. Epub 2022 Aug 18. PMID: 35981020.]