Sandbox Reserved 1771
From Proteopedia
| Line 19: | Line 19: | ||
==Function== | ==Function== | ||
| - | Once bound to an antigen, BCRs undergo a conformational change in the extracellular region. While the exact conformational change is still not known, preliminary studies suggest that there is separation of Fab fragments that opens the binding site within the BCR(ref). This initiates several signal transduction pathways, which are responsible for processing the antigen and initiating the appropriate immune responses. More specifically, the α-β subunit is connected to the phosphorylation of an [https://en.wikipedia.org/wiki/Immunoreceptor_tyrosine-based_activation_motif immunoreceptor tyrosine-based activation motif](ITAM) upon binding. This in turn triggers the activation of kinases downstream that aid in the immune response. BCRs can be oligomeric prior to antigen binding, but once bound become an active monomer. | + | Once bound to an antigen, BCRs undergo a conformational change in the extracellular region. While the exact conformational change is still not known, preliminary studies suggest that there is separation of Fab fragments that opens the binding site within the BCR(ref). This initiates several signal transduction pathways, which are responsible for processing the antigen and initiating the appropriate immune responses. More specifically, the α-β subunit is connected to the phosphorylation of an [https://en.wikipedia.org/wiki/Immunoreceptor_tyrosine-based_activation_motif immunoreceptor tyrosine-based activation motif](ITAM) upon binding. This in turn triggers the activation of kinases downstream that aid in the immune response. BCRs can be oligomeric prior to antigen binding, but once bound become an active monomer. <Ref name="ShenSichen Z"> ShenSichen Z, LiZhengpeng L, Liu W,(2019) Conformational change within the extracellular domain of B cell receptor in B cell activation upon antigen binding [eLife 8:e42271. https://doi.org/10.7554/eLife.42271]</Ref>. |
| + | |||
==Medical Relevancy== | ==Medical Relevancy== | ||
Revision as of 21:31, 29 March 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Contents |
IgM B-cell Receptor
Introduction
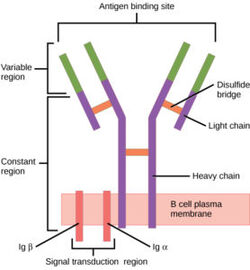
The human B-cell receptor(BCR) is a complex protein made up of three domains: extracellular, transmembrane, and intracellular. While the extracellular region makes up most of the protein, perhaps the most interesting interactions can be found in the transmembrane domain. Unlike other BCRs, the IgM BCR has a specific heavy chain interaction with the α-β subunit of the protein. BCRs are found on the surface of B-cells as membrane bound proteins (ref). In general the role of BCRs is to bind to foreign antigens and initiate the appropriate immune response.
Structure
The BCR consists of a membrane-bound immunoglobulin (mIg) and two signal-transducing subunits, Igα and Igβ. The mIg consists of two heavy chains and two light chains. Antigen binding at the binding site triggers signal transduction by Igα and Igβ. Both consist of an extracellular, Ig-like domain, and an intracellular immunoreceptor tyrosine-based activation motif. [1].
| |||||||||||
Function
Once bound to an antigen, BCRs undergo a conformational change in the extracellular region. While the exact conformational change is still not known, preliminary studies suggest that there is separation of Fab fragments that opens the binding site within the BCR(ref). This initiates several signal transduction pathways, which are responsible for processing the antigen and initiating the appropriate immune responses. More specifically, the α-β subunit is connected to the phosphorylation of an immunoreceptor tyrosine-based activation motif(ITAM) upon binding. This in turn triggers the activation of kinases downstream that aid in the immune response. BCRs can be oligomeric prior to antigen binding, but once bound become an active monomer. [3].
Medical Relevancy
B-cell Formation
The formation of B-cells occurs in the bone marrow from hematopoietic stem cells[4]. Once formed, B-cell receptors are attached to B-cells through the aid of membrane-bound proteins in bone marrow cells. During this process, gene recombination occurs, which allows unique BCRs to become highly specific to different antigens.
Diseases
Therapeutics
References
- ↑ 1.0 1.1 Su Q, Chen M, Shi Y, Zhang X, Huang G, Huang B, Liu D, Liu Z, Shi Y. Cryo-EM structure of the human IgM B cell receptor. Science. 2022 Aug 19;377(6608):875-880. [doi: 10.1126/science.abo3923. Epub 2022 Aug 18. PMID: 35981043.]
- ↑ 2.0 2.1 Tolar P, Pierce SK. Unveiling the B cell receptor structure. Science. 2022 Aug 19;377(6608):819-820. [doi: 10.1126/science.add8065. Epub 2022 Aug 18. PMID: 35981020.]
- ↑ ShenSichen Z, LiZhengpeng L, Liu W,(2019) Conformational change within the extracellular domain of B cell receptor in B cell activation upon antigen binding [eLife 8:e42271. https://doi.org/10.7554/eLife.42271]
- ↑ Althwaiqeb, S. Histology, B Cell Lymphocyte; StatPearls Publishing, 2023.