Sandbox Reserved 1769
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
** Formed by transmembrane helices TM1, TM5, and TM6. | ** Formed by transmembrane helices TM1, TM5, and TM6. | ||
*<b><font color="#0040e0">Core domain</font></b>: Residues 45-154, 209-309 | *<b><font color="#0040e0">Core domain</font></b>: Residues 45-154, 209-309 | ||
| - | **Formed by the packing of a helix bundle of TM2, TM3, and TM4 with another helix bundle of TM7, TM8, and TM9. These two helix bundles are related by pseudo two-fold symmetry.<Ref name="Qi"> Qi X, Li W. Unlocking the secrets to human NTCP structure. Innovation (Camb). 2022 Aug 1;3(5): | + | **Formed by the packing of a helix bundle of TM2, TM3, and TM4 with another helix bundle of TM7, TM8, and TM9. These two helix bundles are related by pseudo two-fold symmetry.<Ref name="Qi"> Qi X, Li W. Unlocking the secrets to human NTCP structure. Innovation (Camb). 2022 Aug 1;3(5):100294. [https://dx.doi.org/10.1016/j.xinn.2022.100294 DOI: 10.1016/j.xinn.2022.100294]. </Ref> |
=== Proline/Glycine Hinge === | === Proline/Glycine Hinge === | ||
| Line 32: | Line 32: | ||
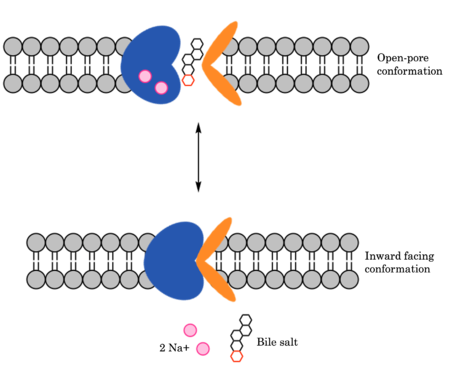
=== Mechanism of HBV/HDV Infection === | === Mechanism of HBV/HDV Infection === | ||
| - | HBV and HDV viruses infect are transported through NTCP via secondary active transport. After binding to NTCP in the open-pore state, the viruses remain bound until low bile salt levels in the blood shift equilibria enough that [https://en.wikipedia.org/wiki/Endocytosis endocytosis] of NTCP occurs. Once in the cell, the viruses dissociate and infect. The exact mechanism of how HBV and HDV bind to NTCP is not certain, although two critical sites have been identified on NTCP: residues 84-87 and 157-165.<ref name="Park" /> Additionally, it has been shown that [https://en.wikipedia.org/wiki/Myristoylation myristoylation] of the HBV/HDV capsid is vital for recognition by NTCP, as well as residues 8-17 on HBV/HDV (sequence: NPLGFFPDHQ). There are two proposed mechanisms for how HBV/HDV binds to NTCP. The first proposes binding of the myristoyl group to the host cell membrane, while residues 8-17 interact with NTCP residues 157-165. The second proposes binding of the myristoyl group with residues 157-165 in the pore.<Ref name="Zhang"> Zhang X, Zhang Q, Peng Q, Zhou J, Liao L, Sun X, Zhang L, Gong T. Hepatitis B virus preS1-derived lipopeptide functionalized liposomes for targeting of hepatic cells. Biomaterials. 2014 Jul;35(23):6130-41 | + | HBV and HDV viruses infect are transported through NTCP via secondary active transport. After binding to NTCP in the open-pore state, the viruses remain bound until low bile salt levels in the blood shift equilibria enough that [https://en.wikipedia.org/wiki/Endocytosis endocytosis] of NTCP occurs. Once in the cell, the viruses dissociate and infect. The exact mechanism of how HBV and HDV bind to NTCP is not certain, although two critical sites have been identified on NTCP: residues 84-87 and 157-165.<ref name="Park" /> Additionally, it has been shown that [https://en.wikipedia.org/wiki/Myristoylation myristoylation] of the HBV/HDV capsid is vital for recognition by NTCP, as well as residues 8-17 on HBV/HDV (sequence: NPLGFFPDHQ). There are two proposed mechanisms for how HBV/HDV binds to NTCP. The first proposes binding of the myristoyl group to the host cell membrane, while residues 8-17 interact with NTCP residues 157-165. The second proposes binding of the myristoyl group with residues 157-165 in the pore.<Ref name="Zhang"> Zhang X, Zhang Q, Peng Q, Zhou J, Liao L, Sun X, Zhang L, Gong T. Hepatitis B virus preS1-derived lipopeptide functionalized liposomes for targeting of hepatic cells. Biomaterials. 2014 Jul;35(23):6130-41. [https://dx.doi.org/10.1016/j.biomaterials.2014.04.037 DOI: 10.1016/j.biomaterials.2014.04.037]. </Ref> |
== Medical Relevance == | == Medical Relevance == | ||
| - | Bile salts are derived from [https://en.wikipedia.org/wiki/Cholesterol cholesterol], and they serve an important role in the mechanical digestion of fats and ultimately facilitate the chemical digestion of lipids. The [https://en.wikipedia.org/wiki/Amphiphile amphipathicity] allows them to do this, solubilizing hydrophobic fats for transport in aqueous bodily fluids. Without bile salts, fats would spontaneously separate out of the aqueous solution in the duodenum and would not be accessible [https://en.wikipedia.org/wiki/Pancreatic_lipase_family#Human_pancreatic_lipase pancreatic lipase] for breakdown. Proper fat digestion requires both pancreatic lipase and bile; thus, NTCP's function in recycling bile salts is critical.<Ref name="Patton"> Patton JS, Carey MC. Watching fat digestion. Science. 1979 Apr 13;204(4389):145-8 | + | Bile salts are derived from [https://en.wikipedia.org/wiki/Cholesterol cholesterol], and they serve an important role in the mechanical digestion of fats and ultimately facilitate the chemical digestion of lipids. The [https://en.wikipedia.org/wiki/Amphiphile amphipathicity] allows them to do this, solubilizing hydrophobic fats for transport in aqueous bodily fluids. Without bile salts, fats would spontaneously separate out of the aqueous solution in the duodenum and would not be accessible [https://en.wikipedia.org/wiki/Pancreatic_lipase_family#Human_pancreatic_lipase pancreatic lipase] for breakdown. Proper fat digestion requires both pancreatic lipase and bile; thus, NTCP's function in recycling bile salts is critical.<Ref name="Patton"> Patton JS, Carey MC. Watching fat digestion. Science. 1979 Apr 13;204(4389):145-8. [https://dx.doi.org/10.1126/science.432636 DOI: 10.1126/science.432636]. </Ref> |
Insight into NTCP's structure and function has implications for therapeutic treatment of HBV/HDV infection. For example, the inhibitory effect of Nb87 on myr-preS1 binding shows potential for therapeutics that stabilize NTCP inward-facing state as [https://en.wikipedia.org/wiki/Allosteric_regulation allosteric inhibitors] of viral cell entry. <Ref name="Zhang" /> | Insight into NTCP's structure and function has implications for therapeutic treatment of HBV/HDV infection. For example, the inhibitory effect of Nb87 on myr-preS1 binding shows potential for therapeutics that stabilize NTCP inward-facing state as [https://en.wikipedia.org/wiki/Allosteric_regulation allosteric inhibitors] of viral cell entry. <Ref name="Zhang" /> | ||
Revision as of 21:53, 29 March 2023
Sodium-taurocholate Co-transporting Polypeptide
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Goutam K, Ielasi FS, Pardon E, Steyaert J, Reyes N. Structural basis of sodium-dependent bile salt uptake into the liver. Nature. 2022 Jun;606(7916):1015-1020. DOI: 10.1038/s41586-022-04723-z.
- ↑ 2.0 2.1 Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. DOI: 10.1038/s41586-022-04845-4.
- ↑ 3.0 3.1 Qi X, Li W. Unlocking the secrets to human NTCP structure. Innovation (Camb). 2022 Aug 1;3(5):100294. DOI: 10.1016/j.xinn.2022.100294.
- ↑ 4.0 4.1 4.2 Liu H, Irobalieva RN, Bang-Sørensen R, Nosol K, Mukherjee S, Agrawal P, Stieger B, Kossiakoff AA, Locher KP. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res. 2022 Aug;32(8):773-776. DOI: 10.1038/s41422-022-00680-4.
- ↑ 5.0 5.1 5.2 5.3 Park JH, Iwamoto M, Yun JH, Uchikubo-Kamo T, Son D, Jin Z, Yoshida H, Ohki M, Ishimoto N, Mizutani K, Oshima M, Muramatsu M, Wakita T, Shirouzu M, Liu K, Uemura T, Nomura N, Iwata S, Watashi K, Tame JRH, Nishizawa T, Lee W, Park SY. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature. 2022 Jun;606(7916):1027-1031. DOI: 10.1038/s41586-022-04857-0.
- ↑ 6.0 6.1 Zhang X, Zhang Q, Peng Q, Zhou J, Liao L, Sun X, Zhang L, Gong T. Hepatitis B virus preS1-derived lipopeptide functionalized liposomes for targeting of hepatic cells. Biomaterials. 2014 Jul;35(23):6130-41. DOI: 10.1016/j.biomaterials.2014.04.037.
- ↑ Patton JS, Carey MC. Watching fat digestion. Science. 1979 Apr 13;204(4389):145-8. DOI: 10.1126/science.432636.
Student Contributors
- Ben Minor
- Maggie Samm
- Zac Stanley