We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1780
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
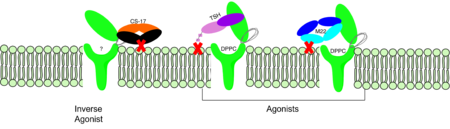
[[Image:Agonist pic.png|450 px|right|thumb|Figure 2: Agonist and antagonist drugs for activating or inactivating the TSHR protein.]] | [[Image:Agonist pic.png|450 px|right|thumb|Figure 2: Agonist and antagonist drugs for activating or inactivating the TSHR protein.]] | ||
| - | |||
| - | ==Introduction== The Thyroid-stimulating hormone receptor (TSHR) is a G protein-coupled receptor (GPCR) that plays a crucial role in regulating the function of the thyroid gland. TSHR is a transmembrane receptor located on the surface of thyroid follicular cells, which are responsible for producing thyroid hormones. TSHR is activated by Thyroid-stimulating hormone (TSH) and triggers a signaling cascade that results in the production and secretion of thyroid hormones. Dysregulation of TSHR can lead to a variety of thyroid disorders, including hyperthyroidism and hypothyroidism. | ||
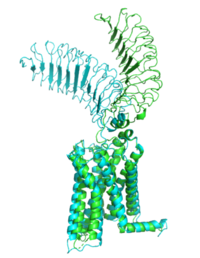
| - | ==Structure== The TSHR protein is a large glycoprotein consisting of 764 amino acids. It is composed of three main domains: the extracellular domain, the transmembrane domain, and the intracellular domain. The extracellular domain of TSHR contains a leucine-rich repeat (LRR) domain, which is responsible for binding to TSH. The transmembrane domain is composed of seven alpha-helices, which span the lipid bilayer of the cell membrane. The intracellular domain is responsible for activating downstream signaling cascades upon TSH binding. | ||
| - | [[File:PDB 1hyt.png|thumb|TSHR protein structure from PDB 1HYT showing the extracellular, transmembrane, and intracellular domains. Click on specific regions to see more details. [[Scene Authoring Tools]] can be used to create hyperlinks.]] | ||
| - | ==Agonists== Several TSHR agonists have been identified, including [[M22]] and [[CS-17]]. M22 is a monoclonal antibody that binds to the extracellular domain of TSHR and mimics the action of TSH, leading to increased production and secretion of thyroid hormones. CS-17 is a small molecule that also binds to the extracellular domain of TSHR and activates downstream signaling pathways. | ||
| - | ==Ligands== Recent studies have identified other compounds that can bind to TSHR and modulate its activity. [[ML109]] is a small molecule that can interact with TSHR and modulate its activity, but the precise mechanism of action is still unknown. [[Dipalmitoylphosphatidylcholine|True DPPC]], a phospholipid found in lung surfactant, has also been shown to bind to TSHR and regulate its activity. However, the physiological relevance of this interaction is still unclear. | ||
| - | ==Function== The TSHR protein plays a critical role in regulating the production and secretion of thyroid hormones. When TSH binds to TSHR on the surface of thyroid follicular cells, it activates a signaling cascade that leads to the production and secretion of thyroid hormones. This process is regulated by a negative feedback loop, where increased levels of thyroid hormones in the bloodstream inhibit the production and secretion of TSH from the pituitary gland. | ||
| - | ==Clinical significance== Dysregulation of TSHR can lead to a variety of thyroid disorders. Hyperthyroidism, a condition characterized by an overactive thyroid gland, can be caused by the overstimulation of TSHR by TSH or TSHR agonists. This can result in an excess of thyroid hormones in the bloodstream, leading to symptoms such as weight loss, rapid heartbeat, and tremors. Hypothyroidism, a condition characterized by an underactive thyroid gland, can be caused by the decreased stimulation of TSHR by TSH. This can result in a deficiency of thyroid hormones in the bloodstream, leading to symptoms such as fatigue, weight gain, and cold intolerance. In addition, mutations in the TSHR gene can lead to inherited thyroid disorders. | ||
| - | ==References== {{reflist|30em}} | ||
| - | ==External links== *[https://proteopedia.org/wiki/index.php/TSH_Receptor TSH Receptor] on Proteopedia. | ||
| - | [[Category:Proteins]] [[Category:G protein-coupled receptors]] | ||
| - | |||
| - | |||
Revision as of 03:40, 30 March 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ Nunez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009 May;42(5):381-95. Epub 2009 Feb 16. PMID:19221175 doi:10.1677/JME-08-0152